
EBK ORGANIC CHEMISTRY
8th Edition
ISBN: 8220102744127
Author: Bruice
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Question
Chapter 15.13, Problem 37P
Interpretation Introduction
Interpretation:
To state the reason for the statement “ The last step in the mechanism of hydroxide-ion promoted hydrolysis of an amide is promoted by amide ion.”
Concept introduction:
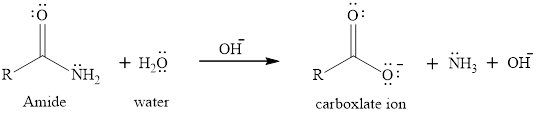
The hydroxide ion promoted hydrolysis of amides is faster reaction as compared to normal hydrolysis reaction. The hydroxide ion participates in the reaction as it is a better nucleophile than water so it attcks the carbonyl carbon and formation of a carboxylate ion and ammonia takes place. The general equation for hydroxide-promoted hydrolysis of amide is written as,
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
3. Assign absolute configuration (Rors) to each chirality center.
a.
H
Nitz
C.
он
b.
0
H-C. C
H
7
C.
་-4
917-417
refs
H
1つ
८
ડુ
d.
Но
f.
-2-
01
Ho
-OH
2HN
How many signals do you expect in the H NMR spectrum for this molecule?
Br
Br
Write the answer below.
Also, in each of the drawing areas below is a copy of the molecule, with Hs shown. In each copy, one of the H atoms is colored red. Highlight in red all other H
atoms that would contribute to the same signal as the H already highlighted red.
Note for advanced students: In this question, any multiplet is counted as one signal.
Number of signals in the 'H NMR spectrum.
For the molecule in the top drawing area, highlight in red any other H atoms that will contribute to
the same signal as the H atom already highlighted red.
If no other H atoms will contribute, check the box at right.
No additional Hs to color in top
molecule
For the molecule in the bottom drawing area, highlight in red any other H atoms that will
contribute to the same signal as the H atom already highlighted red.
If no other H atoms will contribute, check the box at right.
No additional Hs to color in bottom
molecule
In the drawing area below, draw the major products of this organic reaction:
1. NaOH
?
2. CH3Br
If there are no major products, because nothing much will happen to the reactant under these reaction conditions, check the box under the drawing area
instead.
No reaction.
Click and drag to start drawing a
structure.
☐ : A
ค
Chapter 15 Solutions
EBK ORGANIC CHEMISTRY
Ch. 15.1 - The aromas of many flowers and fruits are due to...Ch. 15.1 - Name the following:Ch. 15.1 - Prob. 3PCh. 15.2 - Which is longer, the carbon-oxygen single bond in...Ch. 15.2 - There are three carbon-oxygen bonds in methyl...Ch. 15.2 - Prob. 6PCh. 15.4 - a. What is the product of the reaction of acetyl...Ch. 15.4 - What is the product of an acyl substitution...Ch. 15.5 - a. Which compound has the stretching vibration for...Ch. 15.5 - Using the pKa values listed in Table 15.1, predict...
Ch. 15.5 - Is the following statement true or false? If the...Ch. 15.6 - Starting with acetyl chloride, what neutral...Ch. 15.6 - Prob. 13PCh. 15.7 - Starting with methyl acetate, what neutral...Ch. 15.7 - We saw that it is necessary to use excess amine in...Ch. 15.7 - Prob. 17PCh. 15.7 - Which ester hydrolyzes more rapidly? a. methyl...Ch. 15.7 - a. state three factors that cause the uncatalyzed...Ch. 15.8 - Prob. 21PCh. 15.8 - Using the mechanism for the acid-catalyzed...Ch. 15.8 - Prob. 23PCh. 15.8 - Show the mechanism for the acid-catalyzed...Ch. 15.8 - Prob. 25PCh. 15.8 - Write the mechanism for the acid-catalyzed...Ch. 15.8 - Write the mechanism for the acid-catalyzed...Ch. 15.9 - Prob. 28PCh. 15.9 - Prob. 29PCh. 15.10 - Show how each of the following esters could he...Ch. 15.10 - Prob. 32PCh. 15.11 - Prob. 33PCh. 15.11 - Which of the following reactions leads to the...Ch. 15.12 - Prob. 35PCh. 15.12 - Prob. 36PCh. 15.13 - Prob. 37PCh. 15.14 - Prob. 38PCh. 15.14 - Prob. 39PCh. 15.15 - Prob. 40PCh. 15.15 - Which alkyl halides from the carboxylic acids...Ch. 15.16 - Prob. 43PCh. 15.16 - Prob. 44PCh. 15.16 - Prob. 45PCh. 15.17 - Prob. 46PCh. 15.18 - How could you synthesize the following compounds...Ch. 15 - Prob. 48PCh. 15 - Name the following:Ch. 15 - Prob. 50PCh. 15 - What compound are obtained from the fallowing...Ch. 15 - a. Rank the following esters in order of...Ch. 15 - Because bromocyclohexane is a secondary alkyl...Ch. 15 - a. Which compound would you expect to have a...Ch. 15 - How could you use 1H NMR spectroscopy to...Ch. 15 - Rank the following compounds in order of...Ch. 15 - Prob. 57PCh. 15 - Prob. 58PCh. 15 - Prob. 59PCh. 15 - A compound with molecular formula C5H10O2 gives...Ch. 15 - Prob. 61PCh. 15 - Prob. 62PCh. 15 - Prob. 63PCh. 15 - Prob. 64PCh. 15 - Prob. 65PCh. 15 - Prob. 66PCh. 15 - Two products, A and B, are obtained from the...Ch. 15 - Prob. 68PCh. 15 - Prob. 69PCh. 15 - Prob. 70PCh. 15 - Prob. 71PCh. 15 - Prob. 72PCh. 15 - When treated with an equivalent of methanol,...Ch. 15 - a. Identify the two products obtained from the...Ch. 15 - Prob. 75PCh. 15 - Prob. 76PCh. 15 - a. When a carboxylic acid is dissolved in...Ch. 15 - Prob. 78PCh. 15 - Identity the major and minor products of the...Ch. 15 - When a compound with molecular formula C11H14O2...Ch. 15 - Prob. 81PCh. 15 - Prob. 82PCh. 15 - Prob. 83PCh. 15 - The 1H NMR spectra for two esters with molecular...Ch. 15 - Show how the following compounds could be prepared...Ch. 15 - Prob. 86PCh. 15 - Prob. 87PCh. 15 - The intermediate shown here is formed during the...Ch. 15 - Prob. 89PCh. 15 - Propose a mechanism that accounts for the...Ch. 15 - Catalytic antibodies catalyze a reaction by...Ch. 15 - Prob. 92P
Knowledge Booster
Similar questions
- Predict the major products of the following organic reaction: NC Δ ? Some important Notes: • Draw the major product, or products, of the reaction in the drawing area below. • If there aren't any products, because no reaction will take place, check the box below the drawing area instead. • Be sure to draw bonds carefully to show important geometric relationships between substituents. Note: if your answer contains a complicated ring structure, you must use one of the molecular fragment stamps (available in the menu at right) to enter the ring structure. You can add any substituents using the pencil tool in the usual way. Click and drag to start drawing a structure. Х аarrow_forwardPredict the major products of this organic reaction. Be sure you use dash and wedge bonds to show stereochemistry where it's important. + ☑ OH 1. TsCl, py .... 文 P 2. t-BuO K Click and drag to start drawing a structure.arrow_forwardConsider this organic reaction: ( Draw the major products of the reaction in the drawing area below. If there won't be any major products, because this reaction won't happen at a significant rate, check the box under the drawing area instead. Click and drag to start drawing a structure. Х : а ค 1arrow_forward
- In the drawing area below, draw the major products of this organic reaction: If there are no major products, because nothing much will happen to the reactant under these reaction conditions, check the box under the drawing area instead. 1. NaH 2. CH3Br ? Click and drag to start drawing a structure. No reaction. : ☐ Narrow_forward+ Predict the major product of the following reaction. : ☐ + ☑ ค OH H₂SO4 Click and drag to start drawing a structure.arrow_forwardConsider this organic reaction: ... OH CI Draw the major products of the reaction in the drawing area below. If there won't be any major products, because this reaction won't happen at a significant rate, check the box under the drawing area instead. ☐ No Reaction. Click and drag to start drawing a structure. : аarrow_forward
- Consider the following reactants: Br Would elimination take place at a significant rate between these reactants? Note for advanced students: by significant, we mean that the rate of elimination would be greater than the rate of competing substitution reactions. yes O no If you said elimination would take place, draw the major products in the upper drawing area. If you said elimination would take place, also draw the complete mechanism for one of the major products in the lower drawing area. If there is more than one major product, you may draw the mechanism that leads to any of them. Major Products:arrow_forwardDraw one product of an elimination reaction between the molecules below. Note: There may be several correct answers. You only need to draw one of them. You do not need to draw any of the side products of the reaction. OH + ! : ☐ + Х Click and drag to start drawing a structure.arrow_forwardFind one pertinent analytical procedure for each of following questions relating to food safety analysis. Question 1: The presence of lead, mercury and cadmium in canned tuna Question 2: Correct use of food labellingarrow_forward
- Formulate TWO key questions that are are specifically in relation to food safety. In addition to this, convert these questions into a requirement for chemical analysis.arrow_forwardWhat are the retrosynthesis and forward synthesis of these reactions?arrow_forwardWhich of the given reactions would form meso product? H₂O, H2SO4 III m CH3 CH₂ONa CH3OH || H₂O, H2SO4 CH3 1. LiAlH4, THF 2. H₂O CH3 IVarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you

 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning


Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning