
Chemistry: The Molecular Nature of Matter
7th Edition
ISBN: 9781118516461
Author: Neil D. Jespersen, Alison Hyslop
Publisher: WILEY
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 15, Problem 23RQ
Periodic Trends in the Strength of Acids
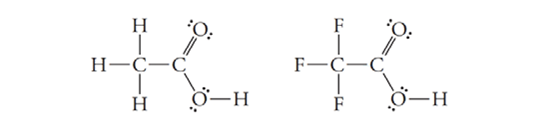
Which of the molecules below is expected to be the stronger Brønsted-Lowry acid? Why?
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Chapter 15 Solutions
Chemistry: The Molecular Nature of Matter
Ch. 15 - Which of the following are conjugate acid-base...Ch. 15 - Write the formula of the conjugate base for each...Ch. 15 - Sodium cyanide solution, when poured into excess...Ch. 15 - One kind of baking powder contains sodium...Ch. 15 - Which of the following are amphoteric and which...Ch. 15 - The anion of sodium monohydrogen phosphate,...Ch. 15 -
Given that is a stronger acid than what is the...Ch. 15 - Given that HClO is a weaker acid than determine...Ch. 15 - Order the following groups of acids from the...Ch. 15 - Using only the periodic cable, choose the stronger...
Ch. 15 - Prob. 11PECh. 15 - Explain why one acid is weaker than the other in...Ch. 15 - In each pair, explain why one is a stronger acid...Ch. 15 - In each pair, explain why one is a weaker acid...Ch. 15 - How would you expect the acidities of the...Ch. 15 - List these acids in terms of increasing acidity:...Ch. 15 - Identify the Lewis acid and Lewis base in each...Ch. 15 - Is the fluoride ion more likely to behave as a...Ch. 15 - Brnsted-Lowry Acids and Bases How is a...Ch. 15 - Brnsted-Lowry Acids and Bases How are the formulas...Ch. 15 - Brnsted-Lowry Acids and Bases Is H2SO4 the...Ch. 15 - Brnsted-Lowry Acids and Bases What is meant by the...Ch. 15 - Brnsted-Lowry Acids and Bases Define the term...Ch. 15 - Strengths of Bronsted-Lowry Acids and Bases
15.6...Ch. 15 - Strengths of Brønsted-Lowry Acids and Bases
15.7...Ch. 15 - Strengths of Brnsted-Lowry Acids and Bases The...Ch. 15 - Strengths of Brnsted-Lowry Acids and Bases...Ch. 15 - Strengths of Brnsted-Lowry Acids and Bases Acetic...Ch. 15 - Strengths of Brnsted-Lowry Acids and Bases Nitric...Ch. 15 - Strengths of Brnsted-Lowry Acids and Bases HCIO4...Ch. 15 - Strengths of Brnsted-Lowry Acids and Bases Formic...Ch. 15 - Periodic Trends in the Strength of Acids Explain...Ch. 15 - Periodic Trends in the Strength of Acids What are...Ch. 15 - Periodic Trends in the Strength of Acids Within...Ch. 15 - Periodic Trends in the Strength of Acids Explain...Ch. 15 - Periodic Trends in the Strength of Acids Within...Ch. 15 - Periodic Trends in the Strength of Acids Explain...Ch. 15 - Periodic Trends in the Strength of Acids Astatine,...Ch. 15 - Periodic Trends in the Strength of Acids
15.21...Ch. 15 - Periodic Trends in the Strength of Acids
15.22...Ch. 15 - Periodic Trends in the Strength of Acids Which of...Ch. 15 - Periodic Trends in the Strength of Acids Which of...Ch. 15 - Lewis Acids and Bases Define Lewis acid and Lewis...Ch. 15 - Lewis Acids and Bases In terms of atomic orbitals,...Ch. 15 - Lewis Acids and Bases
15.27 Explain why the...Ch. 15 - Lewis Acids and Bases Methylamine has the formula...Ch. 15 - Use Lewis structures to show the Lewis acid-base...Ch. 15 - Lewis Acids and Bases
15.30 Explain why the oxide...Ch. 15 - Lewis Acids and Bases The molecule SbF5 is able to...Ch. 15 - Lewis Acids and Bases In the reaction of calcium...Ch. 15 - Acid-Base Properties of the Elements and Their...Ch. 15 - Acid-Base Properties of the Elements and Their...Ch. 15 - Prob. 35RQCh. 15 - Acid-Base Properties of the Elements and Their...Ch. 15 - Acid-Base Properties of the Elements and Their...Ch. 15 - Acid-Base Properties of the Elements and Their...Ch. 15 - Acid-Base Properties of the Elements and Their...Ch. 15 - Prob. 40RQCh. 15 - Acid-Base Properties of the Elements and Their...Ch. 15 - Acid-Base Properties of the Elements and Their...Ch. 15 - Prob. 43RQCh. 15 - Advanced Ceramics and Acid-Base Chemistry What is...Ch. 15 - Advanced Ceramics and Acid-Base Chemistry What is...Ch. 15 - Advanced Ceramics and Acid-Base Chemistry
15.46...Ch. 15 - Advanced Ceramics and Acid-Base Chemistry How does...Ch. 15 - Advanced Ceramics and Acid-Base Chemistry
15.48...Ch. 15 - Brønsted-Lowry Acids and Bases
15.49 Write the...Ch. 15 - Brønsted-Lowry Acids and Bases
15.50 Write the...Ch. 15 - Brønsted-Lowry Acids and Bases
15.51 Write the...Ch. 15 - Brnsted-Lowry Acids and Bases Write the formula...Ch. 15 - Brønsted-Lowry Acids and Bases
15.53 Identify the...Ch. 15 - Brønsted-Lowry Acids and Bases
15.54 Identify the...Ch. 15 - Periodic Trends in the Strengths of Acids Choose...Ch. 15 - Periodic Trends in the Strengths of Acids Choose...Ch. 15 - Choose the stronger acid and give your reason:...Ch. 15 - Choose the stronger acid and give your reason:...Ch. 15 - Choose the stronger acid:...Ch. 15 - Choose the stronger acid:...Ch. 15 - Lewis Acids and Bases Use Lewis symbols co diagram...Ch. 15 - Lewis Acids and Bases Use Lewis symbols to diagram...Ch. 15 - *15.63 Beryllium chloride, , exists in the solid...Ch. 15 - Aluminum chloride, AlCl3, forms molecules with...Ch. 15 - Use Lewis structures to diagram the reaction...Ch. 15 - Use Lewis structures to diagram the reaction...Ch. 15 - Use Lewis structures to show how the following...Ch. 15 - *15.68 Use Lewis structures to show how the...Ch. 15 - Acid-Base Properties of Elements and Their...Ch. 15 - Acid-Base Properties of Elements and Their Oxides...Ch. 15 - Prob. 71RQCh. 15 - Prob. 72RQCh. 15 - What is the formula of the conjugate acid of...Ch. 15 - *15.74 Using liquid ammonia as a solvent, sodium...Ch. 15 - In liquid SO2asasolvent,SOCl2reactswithNa2SO3 in a...Ch. 15 - *15.76 The following space-filling model depicts...Ch. 15 - Which of the following compounds is the stronger...Ch. 15 - Which of the two molecules below is the stronger...Ch. 15 - 15.79 Write equations that illustrate the...Ch. 15 - Hydrogen peroxide is a stronger Brnsted-Lowry acid...Ch. 15 - Sodium hydroxide, NaOH, is basic. Aluminum...Ch. 15 - Hydrazine, N2H4, is a weaker Brnsted-Lowry base...Ch. 15 - Identify the two Brnsted-Lowry acids and two bases...Ch. 15 - In the reaction in the preceding exercise, the...Ch. 15 - How would you expect the degree of ionization of...Ch. 15 - Prob. 86RQCh. 15 - A mixture is prepared containing 0.10 M of each of...Ch. 15 - 15.88 Are all Arrhenius acids Brønsted-Lowry...Ch. 15 - How could you determine whether HBr is a stronger...Ch. 15 - 15.90 Alcohols are organic compounds that have an...Ch. 15 - Acid rain, acid mine runoff, and acid leaching of...Ch. 15 - 15.92 Using just Figure 7.30, find the five most...
Additional Science Textbook Solutions
Find more solutions based on key concepts
A mixed culture of Escherichia coli and Penicillium chrysogenum is inoculated onto the following culture media....
Microbiology: An Introduction
The number of named species is about ________, but the actual number of species on Earth is estimated to be abo...
Biology: Life on Earth with Physiology (11th Edition)
EVOLUTION CONNECTION Describe how gene flow, genetic drift, and natural sclection all can influence macroevolut...
Campbell Biology (11th Edition)
The three dimensional structure of H2S needs to be explained. Concept Introduction: The Lewis structure of an o...
Living By Chemistry: First Edition Textbook
APPLY 1.2 Express the following quantities in scientific notation
using fundamental SI units of mass and lengt...
Chemistry (7th Edition)
19. In FIGURE EX12.19, what magnitude force provides 5.0 N m net torque about the axle?
Physics for Scientists and Engineers: A Strategic Approach, Vol. 1 (Chs 1-21) (4th Edition)
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Summarize the relationship between pKa and base strength by completing the followingsentences: a. For a given base, the higher the pKa of its conjugate acid, the stronger or weaker the base. b. For a given base, the lower the pKa of its conjugate acid, the stronger or weaker the base.arrow_forwardHypofluorous acid, HOF, is known, but fluorous acid, HOFO, is not. Which acid would you expect to be stronger?arrow_forwardThe following reactions illustrate Brnsted acid-base behavior. Complete each equation. a.HI(aq)+?H3O+(aq)+I(aq) b.NH3(l)+?NH4++NH2 c.H2C2O4(aq)+H2O(l)?+HC2O4(aq) d.H2N2O2(aq)+H2O(l)H3O+(aq)+? e.?+H2O(l)H3O+(aq)+CO32(aq)arrow_forward
- Aluminum chloride, AlCl3, behaves more as a molecular compound than an ionic one. This is illustrated in its ability to form a fourth covalent bond with a chloride ion: AlCl3+ClAlCl4. From the Lewis diagram of the aluminum chloride molecule and the electron configuration of the chloride ion, show that this is an acidbase reaction in the Lewis sense, and identify the Lewis acid and the Lewis base. Aluminum chloride is a white solid at room conditions, although it is typically manufactured mixed with some yellow ironIII chloride. Its appearance is that of an ionic compound.arrow_forwarda Write a net ionic equation to show that hypochlorous acid behaves as a Brnsted-Lowry acid in water. b The substance triethanolamine is a weak nitrogen-containing base, like ammonia. Write a net ionic equation to show that triethanolamine, C6H15O3N, behaves as a Brnsted-Lowry base in water.arrow_forwardOxaloacetic acid, which is produced in metabolic reactions, has the structure Would you predict that this acid is a mono-, di-, tri-, or tetraprotic acid? Give your reasoning.arrow_forward
- When a proton becomes bonded to diethyl ether, by way of one of the unshared electron pairs on the oxygen atom, the result is In this structure the oxygen atom owns one electron from each of ____ shared pairs and two electrons from ____ unshared pair. The total number of electrons that belong to oxygen is ____. The formal charge on oxygen is ____. The correct Lewis structure for the conjugate acid of diethyl ether is ___________________________arrow_forwardThe reaction just described is reversible. Deprotonation of the conjugate acid of an organic base by water provides another example of simultaneous making and breaking of sigma bonds. Thus, in the deprotonation of anilinium ion by water, the base is water, which has unshared electrons on the ________ atom. The acid is ________ ion. A pair of ________ electrons on the oxygen atom of water is pushed toward the ________ atom. Simultaneously, the pair of ________ electrons between the hydrogen and ________ atom of the anilinium ion is pushed toward the ________ atom. Thus, the oxygen- ________ sigma bond is made and a hydrogen- ________ sigma bond is broken. The nitrogen atom, which possessed a positive charge, is now ________, and the oxygen atom, which was neutral, now possesses a formal ________ charge.arrow_forwardFor oxyacids, how does acid strength depend on a. the strength of the bond to the acidic hydrogen atom? b. the electronegativity of the element bonded to the oxygen atom that bears the acidic hydrogen? c. the number of oxygen atoms? How does the strength of a conjugate base depend on these factors? What type of solution forms when a nonmetal oxide dissolves in water? Give an example of such an oxide. What type of solution forms when a metal oxide dissolves in water? Give an example of such an oxide.arrow_forward
- When perchloric acid ionizes, it makes the perchlorate ion, ClO4. Draw the Lewis electron dot symbol for the perchlorate ion.arrow_forwardArsenic acid (H3AsO4) is a moderately weak triprotic acid. Write equations showing its stepwise dissociation. Which of the three anions formed in these reactions will be the strongest Brnsted base? Which will be the weakest Brnsted base? Explain your answers.arrow_forwardIn terms of orbitals and electron arrangements, what must be present for a molecule or an ion to act as a Lewis acid? What must be present for a molecule or an ion to act as a Lewis base?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning
Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:9781133109655
Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:Brooks / Cole / Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:9780078746376
Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:Glencoe/McGraw-Hill School Pub Co

Principles of Modern Chemistry
Chemistry
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:Cengage Learning

Chemistry for Today: General, Organic, and Bioche...
Chemistry
ISBN:9781305960060
Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:Cengage Learning


Introductory Chemistry: An Active Learning Approa...
Chemistry
ISBN:9781305079250
Author:Mark S. Cracolice, Ed Peters
Publisher:Cengage Learning
General Chemistry | Acids & Bases; Author: Ninja Nerd;https://www.youtube.com/watch?v=AOr_5tbgfQ0;License: Standard YouTube License, CC-BY