
Chemistry: The Molecular Nature of Matter
7th Edition
ISBN: 9781118516461
Author: Neil D. Jespersen, Alison Hyslop
Publisher: WILEY
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 15, Problem 28RQ
Lewis Acids and Bases
Methylamine has the formula
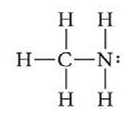
Use Lewis structures to illustrate the reaction of methylaminc with boron trifluoride.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
18
19
20
21
22
23
24
Answer the questions in the table below about the shape of the methyl (CH3) anion.
How many electron groups are around the central carbon atom?
Note: one "electron group" means one lone pair, one single bond,
one double bond, or one triple bond.
0
What phrase best describes the arrangement of these electron
groups around the central carbon atom?
(You may need to use the scrollbar to see all the choices.)
✓ (choose one)
linear
bent
T-shaped
trigonal planar
trigonal pyramidal
square planar
square pyramidal
tetrahedral
sawhorse
trigonal bipyramidal
octahedral
Continue
Two Column N....pages
Kindergarten O....docx
Grey Black and B....pdf
14
431
10
25
nola Mir
Grey Black and B....pdf
26
Ⓒ2022 McGraw Hill LLC. All
When it is strongly heated, ethyl diazoacetate decomposes to give nitrogen gas and a carbene. Draw a Lewis structure of the carbene.
1: Write all the chemical reactions involved in the formation of acid rain from sulfur dioxide (SO2) and rain (H2O).
Chapter 15 Solutions
Chemistry: The Molecular Nature of Matter
Ch. 15 - Which of the following are conjugate acid-base...Ch. 15 - Write the formula of the conjugate base for each...Ch. 15 - Sodium cyanide solution, when poured into excess...Ch. 15 - One kind of baking powder contains sodium...Ch. 15 - Which of the following are amphoteric and which...Ch. 15 - The anion of sodium monohydrogen phosphate,...Ch. 15 -
Given that is a stronger acid than what is the...Ch. 15 - Given that HClO is a weaker acid than determine...Ch. 15 - Order the following groups of acids from the...Ch. 15 - Using only the periodic cable, choose the stronger...
Ch. 15 - Prob. 11PECh. 15 - Explain why one acid is weaker than the other in...Ch. 15 - In each pair, explain why one is a stronger acid...Ch. 15 - In each pair, explain why one is a weaker acid...Ch. 15 - How would you expect the acidities of the...Ch. 15 - List these acids in terms of increasing acidity:...Ch. 15 - Identify the Lewis acid and Lewis base in each...Ch. 15 - Is the fluoride ion more likely to behave as a...Ch. 15 - Brnsted-Lowry Acids and Bases How is a...Ch. 15 - Brnsted-Lowry Acids and Bases How are the formulas...Ch. 15 - Brnsted-Lowry Acids and Bases Is H2SO4 the...Ch. 15 - Brnsted-Lowry Acids and Bases What is meant by the...Ch. 15 - Brnsted-Lowry Acids and Bases Define the term...Ch. 15 - Strengths of Bronsted-Lowry Acids and Bases
15.6...Ch. 15 - Strengths of Brønsted-Lowry Acids and Bases
15.7...Ch. 15 - Strengths of Brnsted-Lowry Acids and Bases The...Ch. 15 - Strengths of Brnsted-Lowry Acids and Bases...Ch. 15 - Strengths of Brnsted-Lowry Acids and Bases Acetic...Ch. 15 - Strengths of Brnsted-Lowry Acids and Bases Nitric...Ch. 15 - Strengths of Brnsted-Lowry Acids and Bases HCIO4...Ch. 15 - Strengths of Brnsted-Lowry Acids and Bases Formic...Ch. 15 - Periodic Trends in the Strength of Acids Explain...Ch. 15 - Periodic Trends in the Strength of Acids What are...Ch. 15 - Periodic Trends in the Strength of Acids Within...Ch. 15 - Periodic Trends in the Strength of Acids Explain...Ch. 15 - Periodic Trends in the Strength of Acids Within...Ch. 15 - Periodic Trends in the Strength of Acids Explain...Ch. 15 - Periodic Trends in the Strength of Acids Astatine,...Ch. 15 - Periodic Trends in the Strength of Acids
15.21...Ch. 15 - Periodic Trends in the Strength of Acids
15.22...Ch. 15 - Periodic Trends in the Strength of Acids Which of...Ch. 15 - Periodic Trends in the Strength of Acids Which of...Ch. 15 - Lewis Acids and Bases Define Lewis acid and Lewis...Ch. 15 - Lewis Acids and Bases In terms of atomic orbitals,...Ch. 15 - Lewis Acids and Bases
15.27 Explain why the...Ch. 15 - Lewis Acids and Bases Methylamine has the formula...Ch. 15 - Use Lewis structures to show the Lewis acid-base...Ch. 15 - Lewis Acids and Bases
15.30 Explain why the oxide...Ch. 15 - Lewis Acids and Bases The molecule SbF5 is able to...Ch. 15 - Lewis Acids and Bases In the reaction of calcium...Ch. 15 - Acid-Base Properties of the Elements and Their...Ch. 15 - Acid-Base Properties of the Elements and Their...Ch. 15 - Prob. 35RQCh. 15 - Acid-Base Properties of the Elements and Their...Ch. 15 - Acid-Base Properties of the Elements and Their...Ch. 15 - Acid-Base Properties of the Elements and Their...Ch. 15 - Acid-Base Properties of the Elements and Their...Ch. 15 - Prob. 40RQCh. 15 - Acid-Base Properties of the Elements and Their...Ch. 15 - Acid-Base Properties of the Elements and Their...Ch. 15 - Prob. 43RQCh. 15 - Advanced Ceramics and Acid-Base Chemistry What is...Ch. 15 - Advanced Ceramics and Acid-Base Chemistry What is...Ch. 15 - Advanced Ceramics and Acid-Base Chemistry
15.46...Ch. 15 - Advanced Ceramics and Acid-Base Chemistry How does...Ch. 15 - Advanced Ceramics and Acid-Base Chemistry
15.48...Ch. 15 - Brønsted-Lowry Acids and Bases
15.49 Write the...Ch. 15 - Brønsted-Lowry Acids and Bases
15.50 Write the...Ch. 15 - Brønsted-Lowry Acids and Bases
15.51 Write the...Ch. 15 - Brnsted-Lowry Acids and Bases Write the formula...Ch. 15 - Brønsted-Lowry Acids and Bases
15.53 Identify the...Ch. 15 - Brønsted-Lowry Acids and Bases
15.54 Identify the...Ch. 15 - Periodic Trends in the Strengths of Acids Choose...Ch. 15 - Periodic Trends in the Strengths of Acids Choose...Ch. 15 - Choose the stronger acid and give your reason:...Ch. 15 - Choose the stronger acid and give your reason:...Ch. 15 - Choose the stronger acid:...Ch. 15 - Choose the stronger acid:...Ch. 15 - Lewis Acids and Bases Use Lewis symbols co diagram...Ch. 15 - Lewis Acids and Bases Use Lewis symbols to diagram...Ch. 15 - *15.63 Beryllium chloride, , exists in the solid...Ch. 15 - Aluminum chloride, AlCl3, forms molecules with...Ch. 15 - Use Lewis structures to diagram the reaction...Ch. 15 - Use Lewis structures to diagram the reaction...Ch. 15 - Use Lewis structures to show how the following...Ch. 15 - *15.68 Use Lewis structures to show how the...Ch. 15 - Acid-Base Properties of Elements and Their...Ch. 15 - Acid-Base Properties of Elements and Their Oxides...Ch. 15 - Prob. 71RQCh. 15 - Prob. 72RQCh. 15 - What is the formula of the conjugate acid of...Ch. 15 - *15.74 Using liquid ammonia as a solvent, sodium...Ch. 15 - In liquid SO2asasolvent,SOCl2reactswithNa2SO3 in a...Ch. 15 - *15.76 The following space-filling model depicts...Ch. 15 - Which of the following compounds is the stronger...Ch. 15 - Which of the two molecules below is the stronger...Ch. 15 - 15.79 Write equations that illustrate the...Ch. 15 - Hydrogen peroxide is a stronger Brnsted-Lowry acid...Ch. 15 - Sodium hydroxide, NaOH, is basic. Aluminum...Ch. 15 - Hydrazine, N2H4, is a weaker Brnsted-Lowry base...Ch. 15 - Identify the two Brnsted-Lowry acids and two bases...Ch. 15 - In the reaction in the preceding exercise, the...Ch. 15 - How would you expect the degree of ionization of...Ch. 15 - Prob. 86RQCh. 15 - A mixture is prepared containing 0.10 M of each of...Ch. 15 - 15.88 Are all Arrhenius acids Brønsted-Lowry...Ch. 15 - How could you determine whether HBr is a stronger...Ch. 15 - 15.90 Alcohols are organic compounds that have an...Ch. 15 - Acid rain, acid mine runoff, and acid leaching of...Ch. 15 - 15.92 Using just Figure 7.30, find the five most...
Additional Science Textbook Solutions
Find more solutions based on key concepts
12. Which of the following experiments could test the hypothesis that bacteria cause ulcers in humans? (Assume ...
Campbell Biology: Concepts & Connections (9th Edition)
Match each of the following items with all the terms it applies to:
Human Physiology: An Integrated Approach (8th Edition)
PRACTICE PROBLEM 14.15 Four benzenoid compounds, all with the formula C7H7Br, gave the following IR peaks in th...
Organic Chemistry
Use a pencil to shade the area between the dashed lines labeled A. This zone represents an impermeable lens of ...
Applications and Investigations in Earth Science (9th Edition)
1.1 Write a one-sentence definition for each of the following:
a. chemistry
b. chemical
Chemistry: An Introduction to General, Organic, and Biological Chemistry (13th Edition)
A mixed culture of Escherichia coli and Penicillium chrysogenum is inoculated onto the following culture media....
Microbiology: An Introduction
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Draw out the Lewis Structure of H3PO4, and consider its major resonance forms. Box the major resonance contributor to the hybrid. List the intermolecular forces the compound would exhibit.arrow_forwardChemical formula of NH4+ and O2-arrow_forwardHow does the acidity of the binary acid of an element vary as a function of the electronegativity of the element? How does this relate to the position of the element in the periodic table?arrow_forward
- Draw the lewis structure of the neutralatom and the ion of nitrogenarrow_forwardIn what sense is acetylene a protonic acid? Explain why its reactions with ammoniacal cuprous chloride and silver nitrate are considered as reactions of an acid?arrow_forwardCalculate the heat of burning ethane (C2H6) in oxygen to give CO₂ and water vapor. What is the minimum energy required to synthesize sulfur dioxide from sulfuric acid?arrow_forward
- Draw acceptable Lewis structure of H4P2O7 and predict the shape.arrow_forwardI need this solution, Calculate the Enthalpy Change (ΔH) from average bond energies, which have been listed below in KJ/mol, for the following reaction and identify the nature of the reaction: CH3COOH + CH3OH → CH3COOCH3 + H2O [C‒H: 413; C‒C: 347; C=O: 745; C=C: 614; Cl‒Cl: 239, C‒O: 358; O‒H: 467arrow_forwardNa2CO3+NaCl givearrow_forward
- the noble gas Xenon acts as a general anesthetic.arrow_forward2 HC= CH + 5 O2 > 4 co2 + 2 h2o What is the energy of the reaction and is it exo- or endothermic?arrow_forwardDescribe how you could separate and purify compound A from a mixture of two neutral compounds (A and B) when A comprises 95% of the total and B the other 5% of the total. Assume that A and B have similar polarities.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax
Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:Cengage Learning


Introductory Chemistry: An Active Learning Approa...
Chemistry
ISBN:9781305079250
Author:Mark S. Cracolice, Ed Peters
Publisher:Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:9781938168390
Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:OpenStax

Introductory Chemistry: A Foundation
Chemistry
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning
INTRODUCTION TO MOLECULAR QUANTUM MECHANICS -Valence bond theory - 1; Author: AGK Chemistry;https://www.youtube.com/watch?v=U8kPBPqDIwM;License: Standard YouTube License, CC-BY