
Concept explainers
(a)
Interpretation:
The structural formula of hemiacetal that is formed when acetaldehyde and methanol reacts has to be drawn.
Concept Introduction:
Aldehydes and ketones react with alcohol to form hemiacetal as the product. This reacts with further molecule of aldehyde or ketone to form acetal.
Hemiacetal is an addition product that is obtained by reaction between aldehyde or ketone with alcohol. The general reaction of hemiacetal formation can be given as,
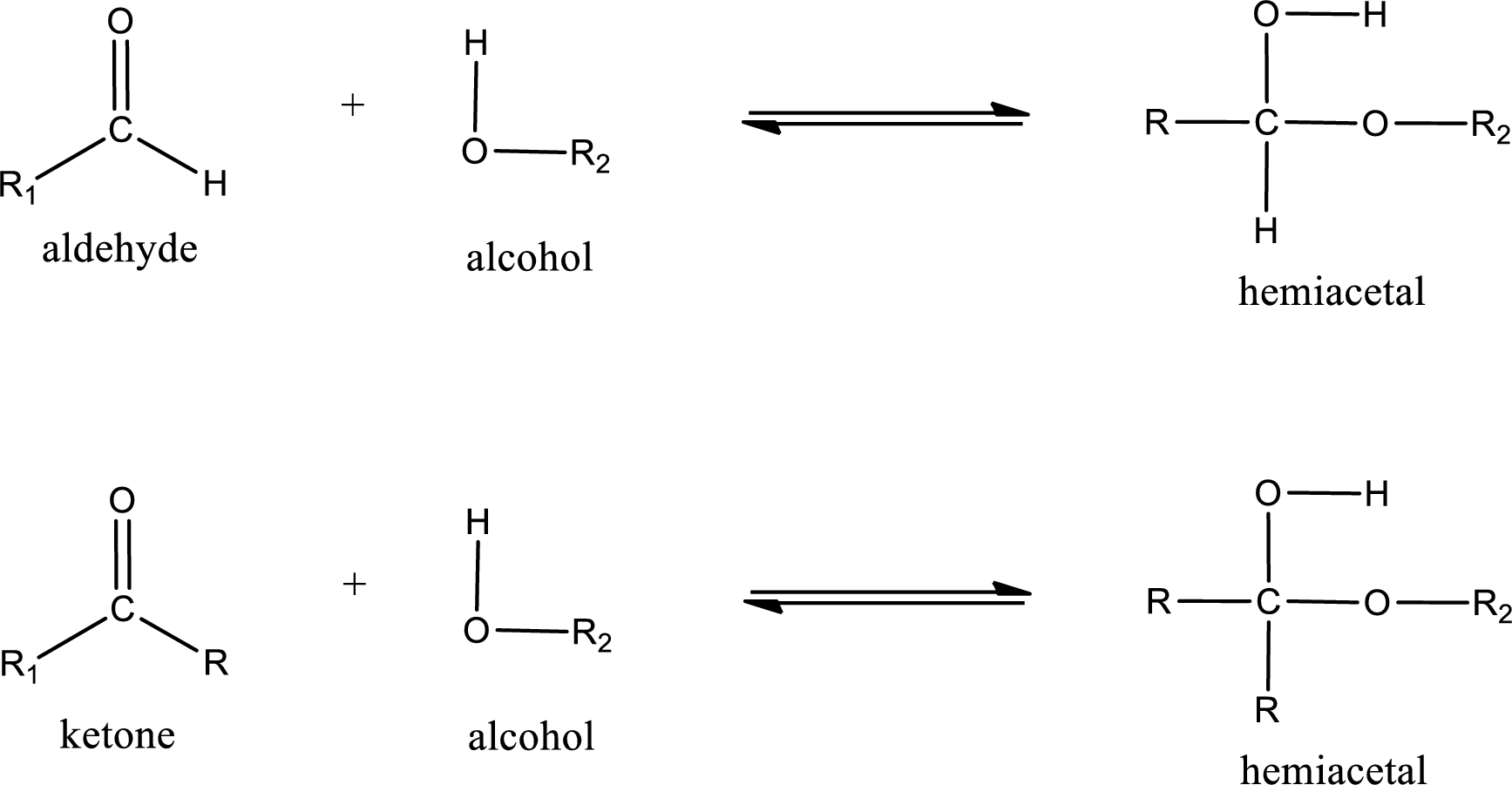
(b)
Interpretation:
The structural formula of hemiacetal that is formed when 2-pentanone and ethyl alcohol reacts has to be drawn.
Concept Introduction:
Aldehydes contain a carbonyl group that is bonded to a hydrogen atom and a carbon atom. Ketones are compounds that contain a carbonyl group bonded to two carbon atoms. Aldehydes and ketones undergo addition reaction across the carbonyl group.
Aldehydes and ketones react with alcohol to form hemiacetal as the product. This reacts with further molecule of aldehyde or ketone to form acetal.
Hemiacetal is an addition product that is obtained by reaction between aldehyde or ketone with alcohol. The general reaction of hemiacetal formation can be given as,
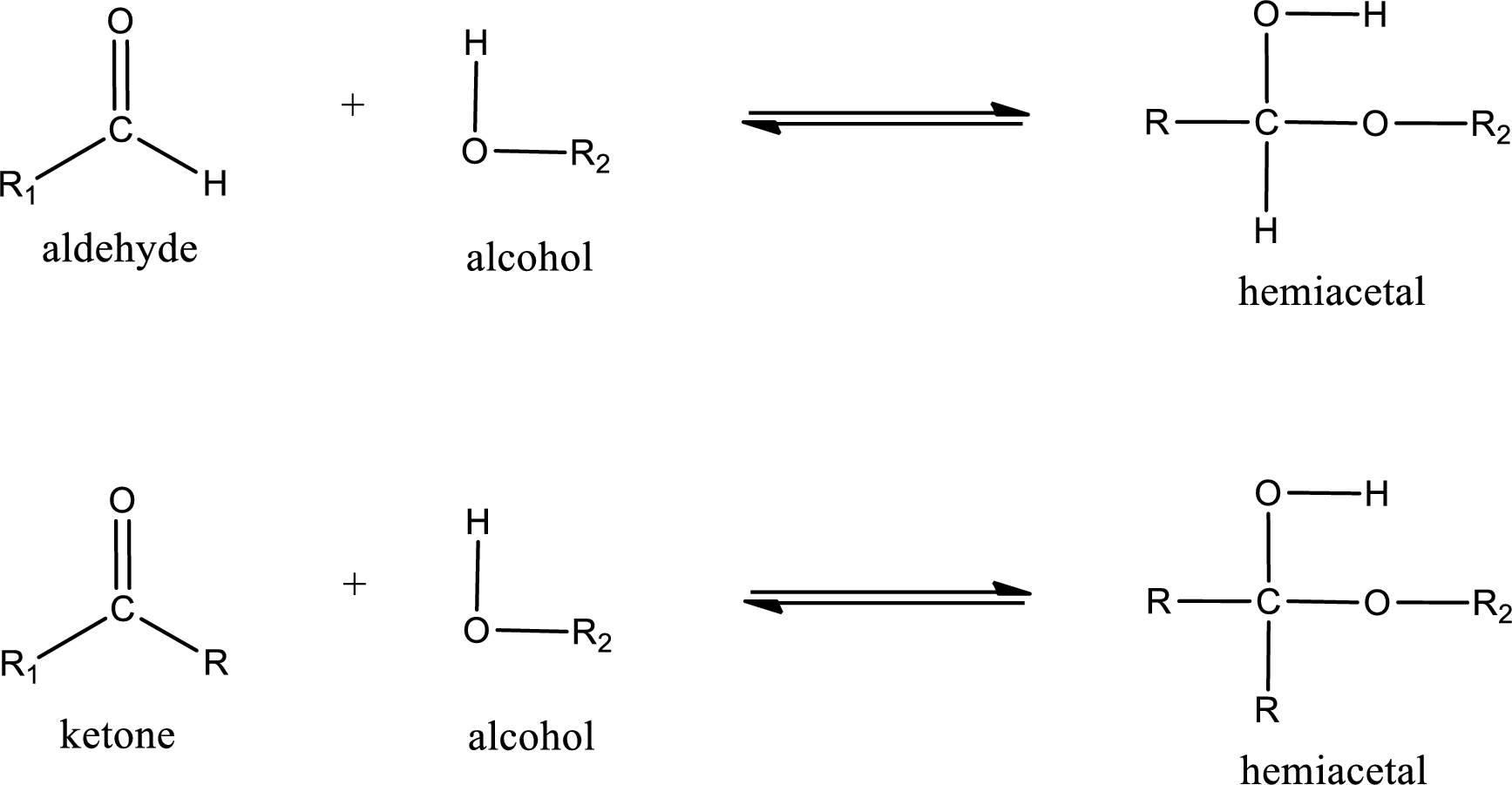
(c)
Interpretation:
The structural formula of hemiacetal that is formed when butanal and isopropyl alcohol reacts has to be drawn.
Concept Introduction:
Aldehydes contain a carbonyl group that is bonded to a hydrogen atom and a carbon atom. Ketones are compounds that contain a carbonyl group bonded to two carbon atoms. Aldehydes and ketones undergo addition reaction across the carbonyl group.
Aldehydes and ketones react with alcohol to form hemiacetal as the product. This reacts with further molecule of aldehyde or ketone to form acetal.
Hemiacetal is an addition product that is obtained by reaction between aldehyde or ketone with alcohol. The general reaction of hemiacetal formation can be given as,
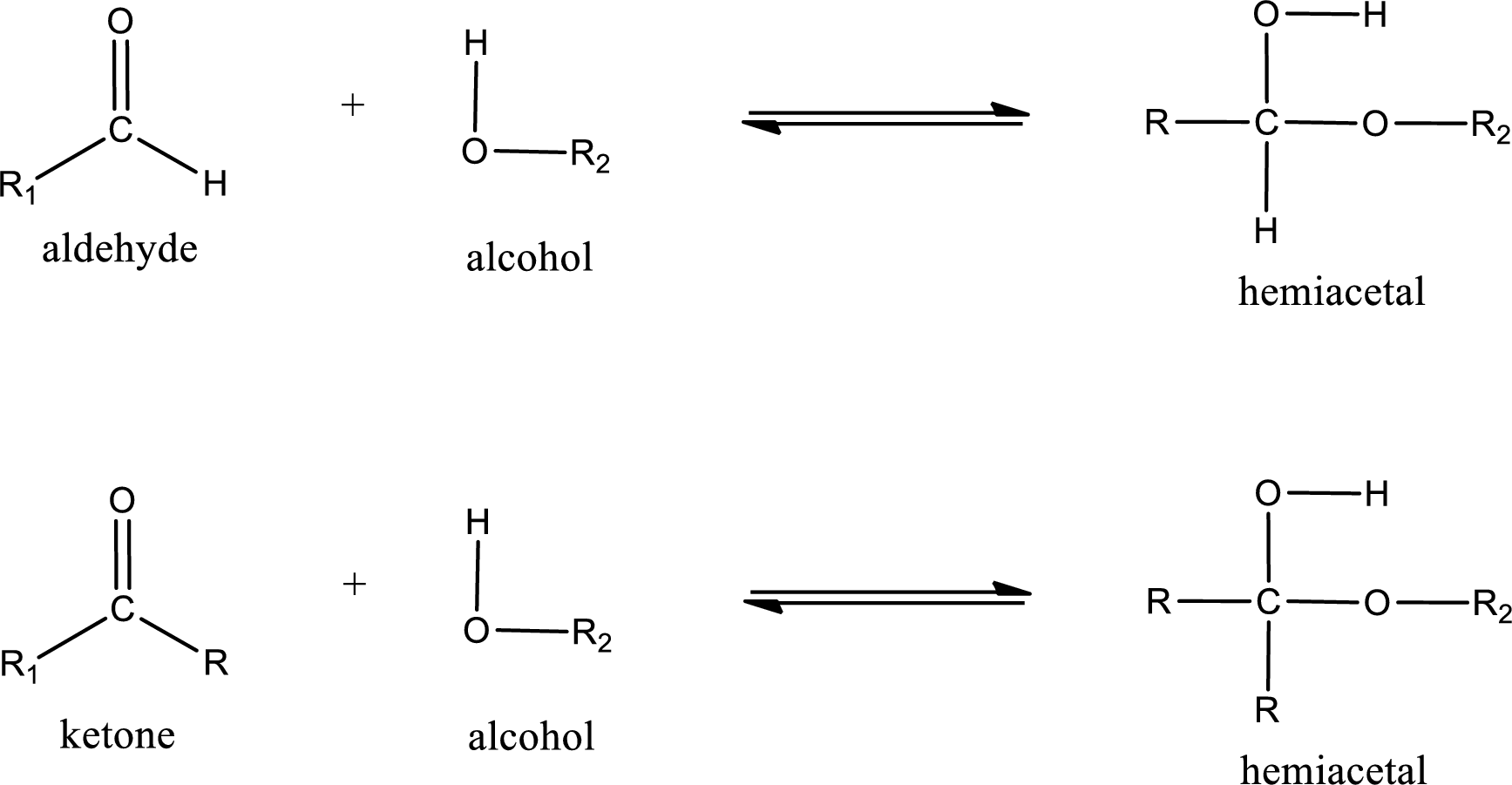
(d)
Interpretation:
The structural formula of hemiacetal that is formed when acetone and ethanol reacts has to be drawn.
Concept Introduction:
Aldehydes contain a carbonyl group that is bonded to a hydrogen atom and a carbon atom. Ketones are compounds that contain a carbonyl group bonded to two carbon atoms. Aldehydes and ketones undergo addition reaction across the carbonyl group.
Aldehydes and ketones react with alcohol to form hemiacetal as the product. This reacts with further molecule of aldehyde or ketone to form acetal.
Hemiacetal is an addition product that is obtained by reaction between aldehyde or ketone with alcohol. The general reaction of hemiacetal formation can be given as,
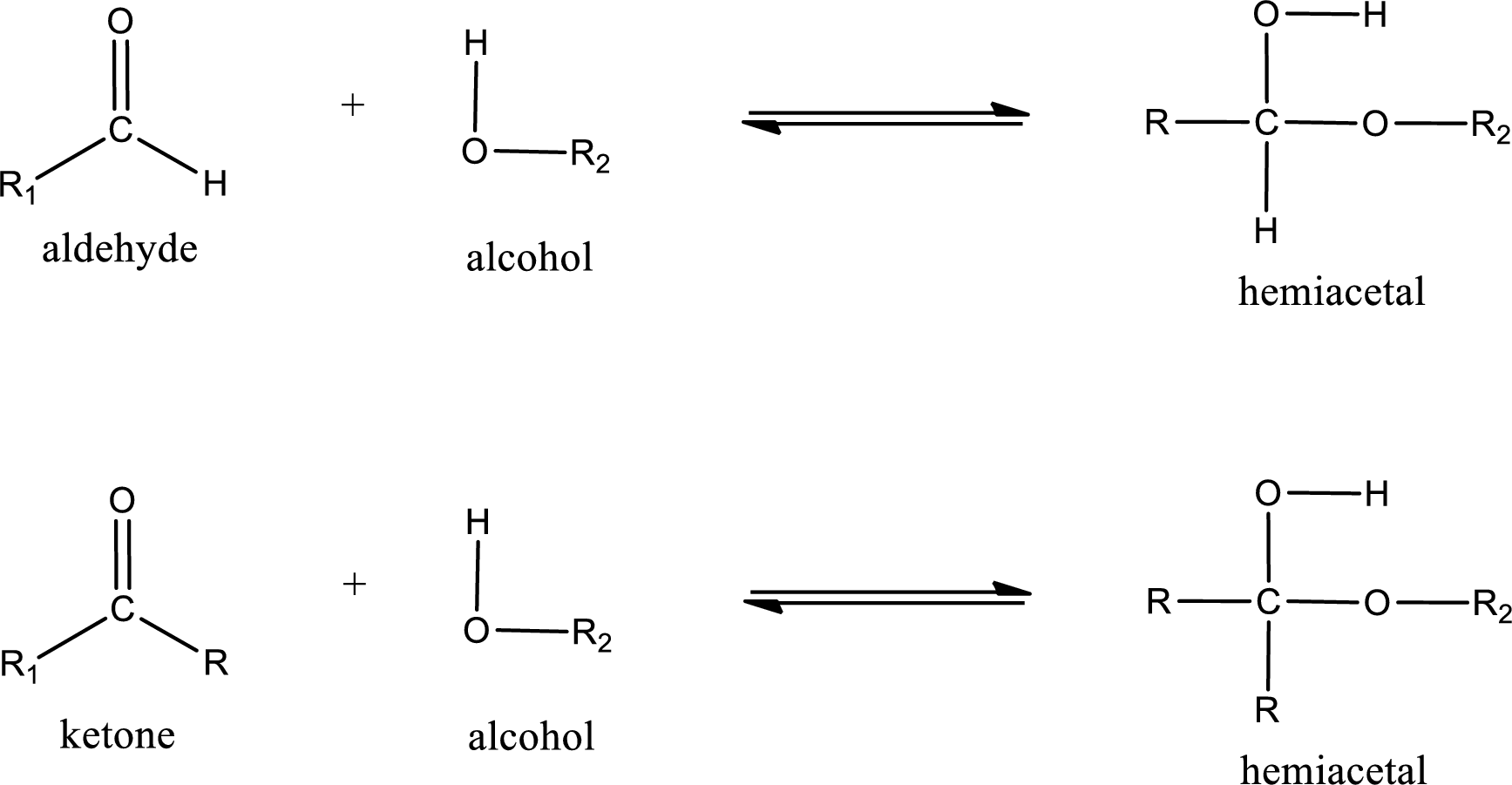
Trending nowThis is a popular solution!

Chapter 15 Solutions
Study Guide with Selected Solutions for Stoker's General, Organic, and Biological Chemistry, 7th
- Neurons and Reflexes 1. Describe the function of the: a) dendrite b) axon c) cell body d) myelin sheath e) nodes of Ranvier f) Schwann cells g) motor neuron, interneuron and sensory neuron 2. List some simple reflexes. Explain why babies are born with simple reflexes. What are they and why are they necessary. 3. Explain why you only feel pain after a few seconds when you touch something very hot but you have already pulled your hand away. 4. What part of the brain receives sensory information? What part of the brain directs you to move your hand away? 5. In your own words describe how the axon fires.arrow_forwardMutations Here is your template DNA strand: CTT TTA TAG TAG ATA CCA CAA AGG 1. Write out the complementary mRNA that matches the DNA above. 2. Write the anticodons and the amino acid sequence. 3. Change the nucleotide in position #15 to C. 4. What type of mutation is this? 5. Repeat steps 1 & 2. 6. How has this change affected the amino acid sequence? 7. Now remove nucleotides 13 through 15. 8. Repeat steps 1 & 2. 9. What type of mutation is this? 0. Do all mutations result in a change in the amino acid sequence? 1. Are all mutations considered bad? 2. The above sequence codes for a genetic disorder called cystic fibrosis (CF). 3. When A is changed to G in position #15, the person does not have CF. When T is changed to C in position #14, the person has the disorder. How could this have originated?arrow_forwardhoose a scientist(s) and research their contribution to our derstanding of DNA structure or replication. Write a one page port and include: their research where they studied and the time period in which they worked their experiments and results the contribution to our understanding of DNA cientists Watson & Crickarrow_forward
- hoose a scientist(s) and research their contribution to our derstanding of DNA structure or replication. Write a one page port and include: their research where they studied and the time period in which they worked their experiments and results the contribution to our understanding of DNA cientists Watson & Crickarrow_forward7. Aerobic respiration of a protein that breaks down into 12 molecules of malic acid. Assume there is no other carbon source and no acetyl-CoA. NADH FADH2 OP ATP SLP ATP Total ATP Show your work using dimensional analysis here: 3arrow_forwardFor each of the following problems calculate the following: (Week 6-3 Video with 6-1 and 6-2) Consult the total catabolic pathways on the last page as a reference for the following questions. A. How much NADH and FADH2 is produced and fed into the electron transport chain (If any)? B. How much ATP is made from oxidative phosphorylation (OP), if any? Feed the NADH and FADH2 into the electron transport chain: 3ATP/NADH, 2ATP/FADH2 C. How much ATP is made by substrate level phosphorylation (SLP)? D. How much total ATP is made? Add the SLP and OP together. 1. Aerobic respiration using 0.5 mole of glucose? NADH FADH2 OP ATP SLP ATP Total ATP Show your work using dimensional analysis here:arrow_forward
- Aerobic respiration of one lipid molecule. The lipid is composed of one glycerol molecule connected to two fatty acid tails. One fatty acid is 12 carbons long and the other fatty acid is 18 carbons long in the figure below. Use the information below to determine how much ATP will be produced from the glycerol part of the lipid. Then, in part B, determine how much ATP is produced from the 2 fatty acids of the lipid. Finally put the NADH and ATP yields together from the glycerol and fatty acids (part A and B) to determine your total number of ATP produced per lipid. Assume no other carbon source is available. 18 carbons fatty acids 12 carbons glycerol . Glycerol is broken down to glyceraldehyde 3-phosphate, a glycolysis intermediate via the following pathway shown in the figure below. Notice this process costs one ATP but generates one FADH2. Continue generating ATP with glyceraldehyde-3-phosphate using the standard pathway and aerobic respiration. glycerol glycerol-3- phosphate…arrow_forwardDon't copy the other answerarrow_forward4. Aerobic respiration of 5 mM acetate solution. Assume no other carbon source and that acetate is equivalent to acetyl-CoA. NADH FADH2 OP ATP SLP ATP Total ATP Show your work using dimensional analysis here: 5. Aerobic respiration of 2 mM alpha-ketoglutaric acid solution. Assume no other carbon source. NADH FADH2 OP ATP Show your work using dimensional analysis here: SLP ATP Total ATParrow_forward
- Biology You’re going to analyze 5 ul of your PCR product(out of 50 ul) on the gel. How much of 6X DNAloading buffer (dye) are you going to mix with yourPCR product to make final 1X concentration ofloading buffer in the PCR product-loading buffermixture?arrow_forwardWrite the assignment on the title "GYMNOSPERMS" focus on the explanation of its important families, characters and reproduction.arrow_forwardAwnser these Discussion Questions Answer these discussion questions and submit them as part of your lab report. Part A: The Effect of Temperature on Enzyme Activity Graph the volume of oxygen produced against the temperature of the solution. How is the oxygen production in 30 seconds related to the rate of the reaction? At what temperature is the rate of reaction the highest? Lowest? Explain. Why might the enzyme activity decrease at very high temperatures? Why might a high fever be dangerous to humans? What is the optimal temperature for enzymes in the human body? Part B: The Effect of pH on Enzyme Activity Graph the volume of oxygen produced against the pH of the solution. At what pH is the rate of reaction the highest? Lowest? Explain. Why does changing the pH affect the enzyme activity? Research the enzyme catalase. What is its function in the human body? What is the optimal pH for the following enzymes found in the human body? Explain. (catalase, lipase (in your stomach),…arrow_forward
 Biology (MindTap Course List)BiologyISBN:9781337392938Author:Eldra Solomon, Charles Martin, Diana W. Martin, Linda R. BergPublisher:Cengage LearningEssentials of Pharmacology for Health ProfessionsNursingISBN:9781305441620Author:WOODROWPublisher:Cengage
Biology (MindTap Course List)BiologyISBN:9781337392938Author:Eldra Solomon, Charles Martin, Diana W. Martin, Linda R. BergPublisher:Cengage LearningEssentials of Pharmacology for Health ProfessionsNursingISBN:9781305441620Author:WOODROWPublisher:Cengage Anatomy & PhysiologyBiologyISBN:9781938168130Author:Kelly A. Young, James A. Wise, Peter DeSaix, Dean H. Kruse, Brandon Poe, Eddie Johnson, Jody E. Johnson, Oksana Korol, J. Gordon Betts, Mark WomblePublisher:OpenStax College
Anatomy & PhysiologyBiologyISBN:9781938168130Author:Kelly A. Young, James A. Wise, Peter DeSaix, Dean H. Kruse, Brandon Poe, Eddie Johnson, Jody E. Johnson, Oksana Korol, J. Gordon Betts, Mark WomblePublisher:OpenStax College





