
Concept explainers
(a)
Interpretation: To characterize
Concept introduction: Transamination reaction is a biochemical reaction that involves the transfer of an amino group. In transamination reaction exchange of an amino group from an
A biochemical reaction in which an
A general oxidative deamination reaction is as follows:
(a)
Answer to Problem 15.45EP
Explanation of Solution
In both transamination and oxidative deamination reaction exchange of an amino group from an
(b)
Interpretation: To characterize glutamate as a possible reactant, product, or enzyme involved in transamination, oxidative deamination, or both transamination and oxidative deamination.
Concept introduction: Transamination reaction is a biochemical reaction that involves the transfer of an amino group. In transamination reaction exchange of an amino group from an
A biochemical reaction in which an
A general oxidative deamination reaction is as follows:
(b)
Answer to Problem 15.45EP
Glutamate acts as a reactant in both transamination and oxidative deamination reaction.
Explanation of Solution
In both transamination and oxidative deamination reaction exchange of an amino group from an
Glutamate is an amino acid thus acts as a reactant in both transamination and oxidative deamination reaction to give corresponding keto acid
(c)
Interpretation: To characterize glutamate dehydrogenase as a possible reactant, product, or enzyme involved in transamination, oxidative deamination, or both transamination and oxidative deamination.
Concept introduction: Transamination reaction is a biochemical reaction that involves the transfer of an amino group. In transamination reaction exchange of an amino group from an
A biochemical reaction in which an
A general oxidative deamination reaction is as follows:
(c)
Answer to Problem 15.45EP
Glutamate dehydrogenase is the enzyme involved in the oxidative deamination reaction of glutamate to give
Explanation of Solution
Oxidative deamination reaction of glutamate requires dehydrogenase enzyme. It is an oxidoreductase enzyme and works with either
(d)
Interpretation: To characterize
Concept introduction: Transamination reaction is a biochemical reaction that involves the transfer of an amino group. In transamination reaction exchange of an amino group from an
A biochemical reaction in which an
A general oxidative deamination reaction is as follows:
(d)
Answer to Problem 15.45EP
The ammonium ion is one of the products obtained in oxidative deamination reaction of glutamate.
Explanation of Solution
Glutamate is an
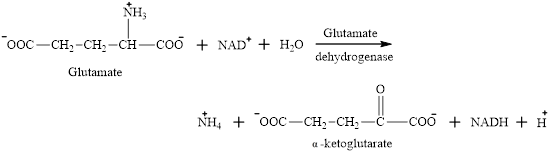
The ammonium ion formed is toxic if build up in the body. Ammonium ion acts as the “nitrogen carrier” for further reactions.
Want to see more full solutions like this?
Chapter 15 Solutions
Organic And Biological Chemistry
- 3. Use Kapustinskii's equation and data from Table 4.10 in your textbook to calculate lattice energies of Cu(OH)2 and CuCO3 (4 points)arrow_forward2. Copper (II) oxide crystalizes in monoclinic unit cell (included below; blue spheres 2+ represent Cu²+, red - O²-). Use Kapustinski's equation (4.5) to calculate lattice energy for CuO. You will need some data from Resource section of your textbook (p.901). (4 points) CuOarrow_forwardWhat is the IUPAC name of the following compound? OH (2S, 4R)-4-chloropentan-2-ol O (2R, 4R)-4-chloropentan-2-ol O (2R, 4S)-4-chloropentan-2-ol O(2S, 4S)-4-chloropentan-2-olarrow_forward
- Use the reaction coordinate diagram to answer the below questions. Type your answers into the answer box for each question. (Watch your spelling) Energy A B C D Reaction coordinate E A) Is the reaction step going from D to F endothermic or exothermic? A F G B) Does point D represent a reactant, product, intermediate or transition state? A/ C) Which step (step 1 or step 2) is the rate determining step? Aarrow_forward1. Using radii from Resource section 1 (p.901) and Born-Lande equation, calculate the lattice energy for PbS, which crystallizes in the NaCl structure. Then, use the Born-Haber cycle to obtain the value of lattice energy for PbS. You will need the following data following data: AH Pb(g) = 196 kJ/mol; AHƒ PbS = −98 kJ/mol; electron affinities for S(g)→S¯(g) is -201 kJ/mol; S¯(g) (g) is 640kJ/mol. Ionization energies for Pb are listed in Resource section 2, p.903. Remember that enthalpies of formation are calculated beginning with the elements in their standard states (S8 for sulfur). The formation of S2, AHF: S2 (g) = 535 kJ/mol. Compare the two values, and explain the difference. (8 points)arrow_forwardIn the answer box, type the number of maximum stereoisomers possible for the following compound. A H H COH OH = H C Br H.C OH CHarrow_forward
- 7. Magnesium is found in nature in the form of carbonates and sulfates. One of the major natural sources of zinc is zinc blende (ZnS). Use relevant concepts of acid-base theory to explain this combination of cations and anions in these minerals. (2 points)arrow_forward6. AlF3 is insoluble in liquid HF but dissolves if NaF is present. When BF3 is added to the solution, AlF3 precipitates. Write out chemical processes and explain them using the principles of Lewis acid-base theory. (6 points)arrow_forward5. Zinc oxide is amphoteric. Write out chemical reactions for dissolution of ZnO in HCl(aq) and in NaOH(aq). (3 points)arrow_forward
 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,




