
Concept explainers
(a)
Interpretation: To determine whether denaturation of protein is associated with the (1) mouth, (2) stomach, (3) small intestine or (4) intestinal lining.
Concept introduction: Proteins are natural biopolymers. Amino acids are the main building block of protein molecules. A large number of amino acids condense together to form a polypeptide chain. A large polypeptide chain is called protein.
Denaturation of protein is defined as the process in which the quaternary, tertiary and secondary structure of the protein is disrupted except the primary structure with the help of external sources. Denaturation disrupts the intermolecular forces responsible for the protein structure. Denaturation of proteins can be carried out by treatment of protein with strong acid or bases, solvents, reducing and oxidizing agents.
(a)
Answer to Problem 15.1EP
Protein is denatured in the stomach.
Explanation of Solution
Reason for correct option:
Denaturation of proteins can be effected by treatment of protein with strong acid or bases, solvents, reducing and oxidizing agents. When dietary protein enters the stomach it triggers the release of gastrin hormone by mucosa cells of the stomach. Gastrin secretion, in turn, causes secretion of hydrochloric acid. Hydrochloric acid is a strong acid and thus result in the denaturation of the protein.
Reason for incorrect option:
Digestion of protein starts in the stomach, not in the mouth. Salive present in the mouth just helps to swallow down the food it does not affect the protein. Hence, denaturation of protein is not associated with the mouth.
In the small intestine, bicarbonate ion is secreted by secretin hormone. Bicarbonate is weakly basic and thus does not denatures protein rather in small intestine peptide bonds are hydrolyzed by the pancreatic enzymes. Hence, denaturation of protein is not associated with the small intestine.
Protein digestion is completed in the small intestine. The free amino acids released are absorbed through the intestinal lining into the bloodstream. Hence, denaturation of protein is not associated with the intestinal lining.
(b)
Interpretation: To determine whether active trypsin is associated with the (1) mouth, (2) stomach, (3) small intestine or (4) intestinal lining.
Concept introduction: Proteins are natural biopolymers. Amino acids are the main building block of protein molecules. A large number of amino acids condense together to form a polypeptide chain. A large polypeptide chain is called protein. The digestion of dietary protein starts in the stomach and is completed in the small intestine. It does not start in the mouth because saliva present in the mouth does not contain an effective enzyme to break down the protein. The flow chart for protein digestion in the human body is as follows:
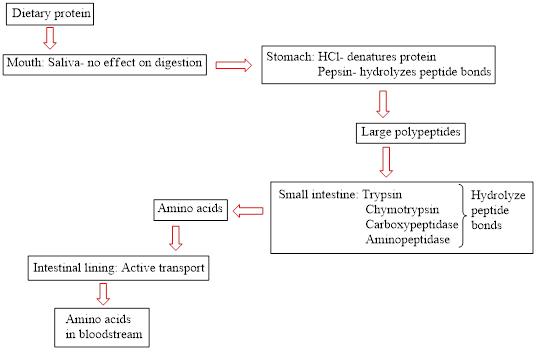
(b)
Answer to Problem 15.1EP
Trypsin is active in the small intestine.
Explanation of Solution
Reason for correct option:
Trypsin is an example of a proteolytic enzyme. The basic environment in the small intestine stimulates the production of pancreatic digestive enzyme trypsin. Trypsin is active in the small intestine and attacks the peptide bond of the proteins.
Reason for incorrect option:
The only enzyme present in the mouth is salivary amylase. Pepsin is present in the stomach and no enzyme is present in the intestinal lining.
(c)
Interpretation: To determine whether active breaking of peptide bonds is completed in the (1) mouth, (2) stomach, (3) small intestine or (4) intestinal lining.
Concept introduction: Proteins are natural biopolymers. Amino acids are the main building block of protein molecules. A large number of amino acids condense together to form a polypeptide chain. A large polypeptide chain is called protein. The digestion of dietary protein starts in the stomach and is completed in the small intestine. It does not start in the mouth because saliva present in the mouth does not contain an effective enzyme to break down the protein. The flow chart for protein digestion in the human body is as follows:

(c)
Answer to Problem 15.1EP
The breaking of peptide bonds is completed in the small intestine.
Explanation of Solution
Reason for correct option:
The partially digested polypeptides are passed into the small intestine from the stomach. In small intestine trypsin, chymotrypsin, carboxypeptidase, and aminopeptidase digestive enzyme are present. These attack and hydrolyzes the peptide bond of the protein and breaks the long polypeptide chains into free amino acid residues resulting in the complete digestion or breaking of the peptide bonds.
Reason for incorrect option:
Digestion of protein starts in the stomach, not in the mouth. In the stomach hydrochloric acid present denatures or unwind the globular protein and the enzyme pepsin hydrolyzes only
(d)
Interpretation: To determine whether hydrochloric acid is secreted in the (1) mouth, (2) stomach, (3) small intestine or (4) intestinal lining.
Concept introduction: Proteins are natural biopolymers. Amino acids are the main building block of protein molecules. A large number of amino acids condense together to form a polypeptide chain. A large polypeptide chain is called protein. The digestion of dietary protein starts in the stomach and is completed in the small intestine. It does not start in the mouth because saliva present in the mouth does not contain an effective enzyme to break down the protein. The flow chart for protein digestion in the human body is as follows:

(d)
Answer to Problem 15.1EP
Hydrochloric acid is secreted in the stomach.
Explanation of Solution
Reason for correct option:
Hydrochloric acid secretion is stimulated by the production of gastrin hormone which in turn is released by mucosa cells of the stomach. Thus, hydrochloric acid is secreted in the stomach.
Reason for incorrect option:
Hydrochloric acid is secreted in the stomach and is responsible for denaturation of the protein. Protein denaturation is the first step of protein digestion that occurs in the stomach. Hence, hydrochloric acid is not associated with the mouth, small intestine or intestinal lining.
Want to see more full solutions like this?
Chapter 15 Solutions
Organic And Biological Chemistry
- Indicate the product(s) B and C that are formed in the reaction: HN' OCH HC1 B + mayoritario C minoritario OCH3arrow_forwardIndicate the product(s) that are formed in the reaction: NH-NH, OCH3 -H₂O OCH3arrow_forward21.38 Arrange the molecules in each set in order of increasing acidity (from least acidic to most acidic). OH OH SH NH2 8 NH3 OH (b) OH OH OH (c) & & & CH3 NO2 21.39 Explain the trends in the acidity of phenol and the monofluoro derivatives of phenol. OH OH OH OH PK 10.0 PK 8.81 PK 9.28 PK 9.81arrow_forward
- identify which spectrum is for acetaminophen and which is for phenacetinarrow_forwardThe Concept of Aromaticity 21.15 State the number of 2p orbital electrons in each molecule or ion. (a) (b) (e) (f) (c) (d) (h) (i) DA (k) 21.16 Which of the molecules and ions given in Problem 21.15 are aromatic according to the Hückel criteria? Which, if planar, would be antiaromatic? 21.17 Which of the following structures are considered aromatic according to the Hückel criteria? ---0-0 (a) (b) (c) (d) (e) (h) H -H .8.0- 21.18 Which of the molecules and ions from Problem 21.17 have electrons donated by a heteroatom?arrow_forward1. Show the steps necessary to make 2-methyl-4-nonene using a Wittig reaction. Start with triphenylphosphine and an alkyl halide. After that you may use any other organic or inorganic reagents. 2. Write in the product of this reaction: CH3 CH₂ (C6H5)₂CuLi H₂O+arrow_forward
- 3. Name this compound properly, including stereochemistry. H₂C H3C CH3 OH 4. Show the step(s) necessary to transform the compound on the left into the acid on the right. Bri CH2 5. Write in the product of this LiAlH4 Br H₂C OHarrow_forwardWhat are the major products of the following reaction? Please provide a detailed explanation and a drawing to show how the reaction proceeds.arrow_forwardWhat are the major products of the following enolate alkylation reaction? Please include a detailed explanation as well as a drawing as to how the reaction proceeds.arrow_forward
- A block of zinc has an initial temperature of 94.2 degrees celcius and is immererd in 105 g of water at 21.90 degrees celcius. At thermal equilibrium, the final temperature is 25.20 degrees celcius. What is the mass of the zinc block? Cs(Zn) = 0.390 J/gxdegrees celcius Cs(H2O) = 4.18 J/gx degrees celcusarrow_forwardPotential Energy (kJ) 1. Consider these three reactions as the elementary steps in the mechanism for a chemical reaction. AH = -950 kJ AH = 575 kJ (i) Cl₂ (g) + Pt (s) 2C1 (g) + Pt (s) Ea = 1550 kJ (ii) Cl (g)+ CO (g) + Pt (s) → CICO (g) + Pt (s) (iii) Cl (g) + CICO (g) → Cl₂CO (g) Ea = 2240 kJ Ea = 2350 kJ AH = -825 kJ 2600 2400 2200 2000 1800 1600 1400 1200 1000 a. Draw the potential energy diagram for the reaction. Label the data points for clarity. The potential energy of the reactants is 600 kJ 800 600 400 200 0 -200- -400 -600- -800- Reaction Progressarrow_forwardCan u help me figure out the reaction mechanisms for these, idk where to even startarrow_forward
 General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning, Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning Chemistry In FocusChemistryISBN:9781305084476Author:Tro, Nivaldo J., Neu, Don.Publisher:Cengage Learning
Chemistry In FocusChemistryISBN:9781305084476Author:Tro, Nivaldo J., Neu, Don.Publisher:Cengage Learning





