
Concept explainers
(a)
Interpretation:
To indicate whether B vitamin riboflavin is involved as a cofactor in the processes of (1) transamination, (2) oxidative deamination, (3) urea cycle, (4) carbon skeleton degradation to CAC intermediates, or (5) carbon skeleton degradation to non-CAC intermediates.
Concept introduction:
Cofactors are non-protein organic compounds that are used along with the enzymes and help to carry forward the reaction. The coenzymes containing B-vitamin serve as temporary carriers of atoms or
Transamination reaction is a biochemical reaction that involves the transfer of an amino group. In transamination reaction exchange of an amino group from an
A biochemical reaction in which an
A urea cycle is a cyclic biochemical pathway that involves the production of urea using ammonium ions and aspartate molecules as nitrogen sources. The reactants in the formation of carbamoyl phosphate are ammonium ion, water, and carbon dioxide. The desired product of the urea cycle is urea.
There are 20 standard amino acids. Each amino acid has a different carbon skeleton and has a different degradation pathway for its carbon skeleton.
(a)
Answer to Problem 15.116EP
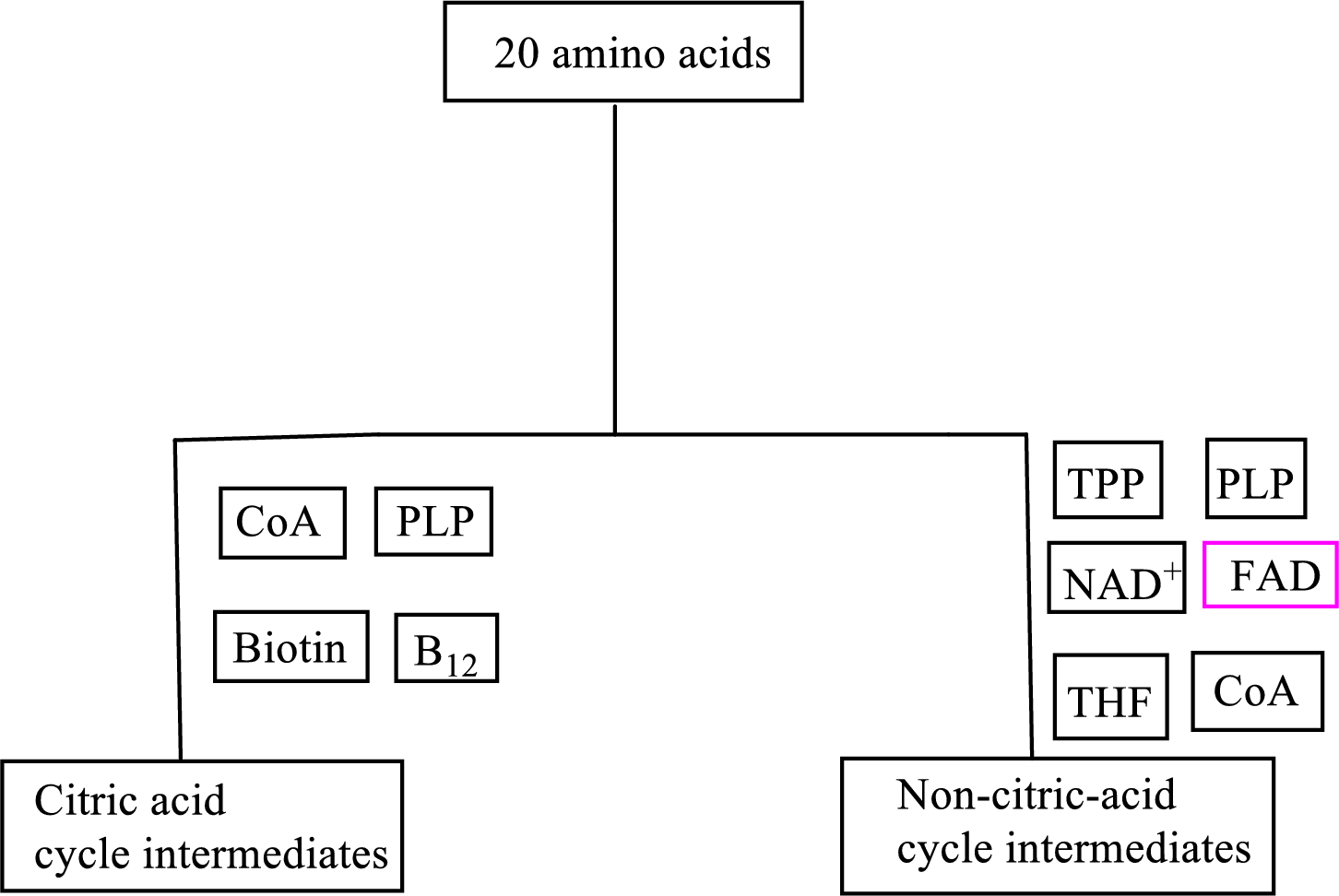
B vitamin riboflavin is involved as a cofactor in carbon skeleton degradation to non-CAC intermediates.
Explanation of Solution
Coenzyme flavin adenine dinucleotide
Flavin adenine dinucleotide
(b)
Interpretation:
To indicate whether B vitamin thiamin is involved as a cofactor in the processes of (1) transamination, (2) oxidative deamination, (3) urea cycle, (4) carbon skeleton degradation to CAC intermediates, or (5) carbon skeleton degradation to non-CAC intermediates.
Concept introduction:
Cofactors are non-protein organic compounds that are used along with the enzymes and help to carry forward the reaction. The coenzymes containing B-vitamin serve as temporary carriers of atoms or functional groups in the redox and group transfer reactions associated with the metabolism of ingested food in order to obtain energy from the food.
Transamination reaction is a biochemical reaction that involves the transfer of an amino group. In transamination reaction exchange of an amino group from an
A biochemical reaction in which an
A urea cycle is a cyclic biochemical pathway that involves the production of urea using ammonium ions and aspartate molecules as nitrogen sources. The reactants in the formation of carbamoyl phosphate are ammonium ion, water, and carbon dioxide. The desired product of the urea cycle is urea.
There are 20 standard amino acids. Each amino acid has a different carbon skeleton and has a different degradation pathway for its carbon skeleton.
(b)
Answer to Problem 15.116EP
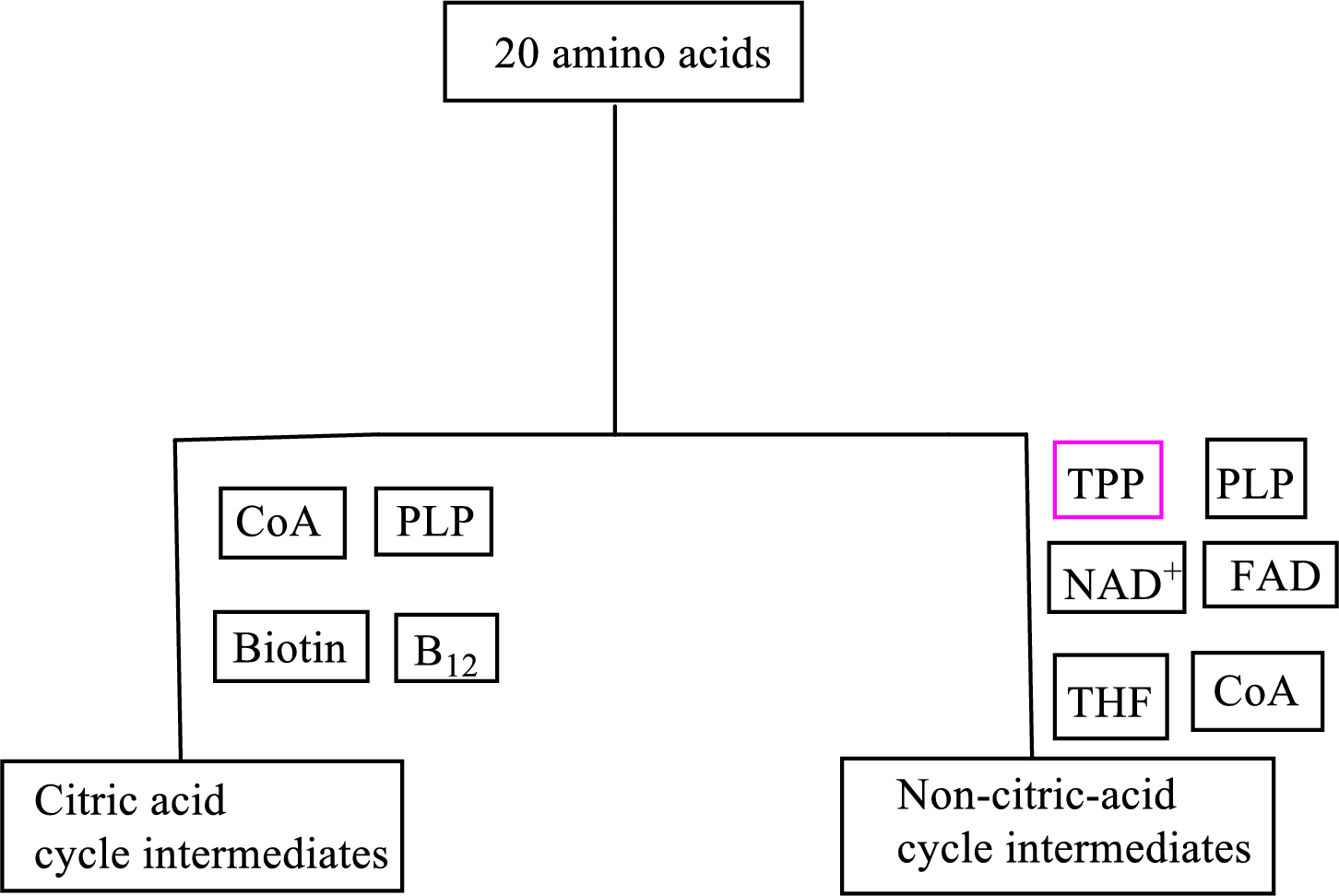
B vitamin thiamin is involved as a cofactor in carbon skeleton degradation to non-CAC intermediates.
Explanation of Solution
Coenzyme thiamin pyrophosphate
Thiamin pyrophosphate
(c)
Interpretation:
To indicate whether B vitamin pantothenic is involved as a cofactor in the processes of (1) transamination, (2) oxidative deamination, (3) urea cycle, (4) carbon skeleton degradation to CAC intermediates, or (5) carbon skeleton degradation to non-CAC intermediates.
Concept introduction:
Cofactors are non-protein organic compounds that are used along with the enzymes and help to carry forward the reaction. The coenzymes containing B-vitamin serve as temporary carriers of atoms or functional groups in the redox and group transfer reactions associated with the metabolism of ingested food in order to obtain energy from the food.
Transamination reaction is a biochemical reaction that involves the transfer of an amino group. In transamination reaction exchange of an amino group from an
A biochemical reaction in which an
A urea cycle is a cyclic biochemical pathway that involves the production of urea using ammonium ions and aspartate molecules as nitrogen sources. The reactants in the formation of carbamoyl phosphate are ammonium ion, water, and carbon dioxide. The desired product of the urea cycle is urea.
There are 20 standard amino acids. Each amino acid has a different carbon skeleton and has a different degradation pathway for its carbon skeleton.
(c)
Answer to Problem 15.116EP
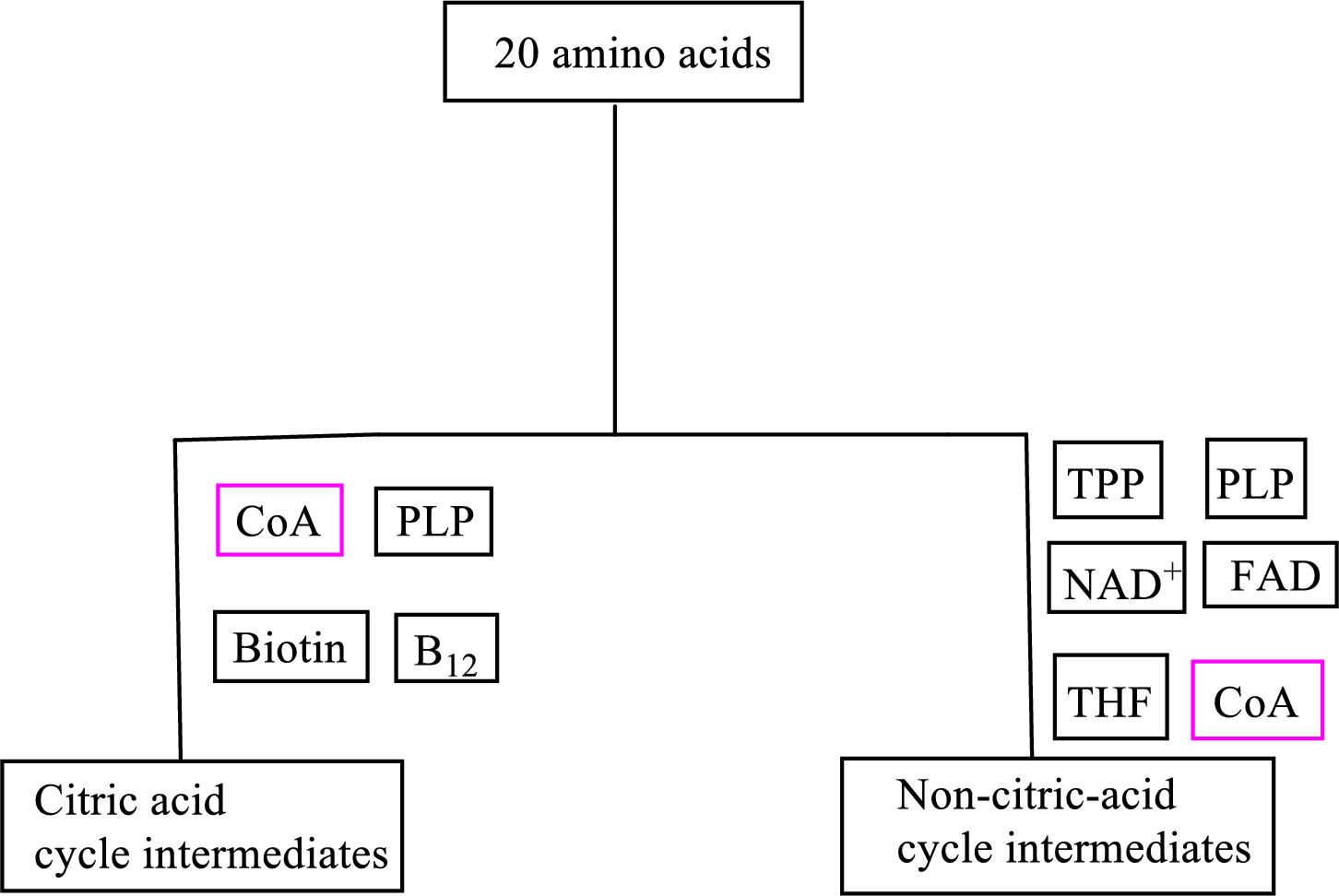
B vitamin pantothenic acid is involved as a cofactor in carbon skeleton degradation to non-CAC and CAC intermediates.
Explanation of Solution
Coenzyme A
Coenzyme A
(d)
Interpretation:
To indicate whether B vitamin
Concept introduction:
Cofactors are non-protein organic compounds that are used along with the enzymes and help to carry forward the reaction. The coenzymes containing B-vitamin serve as temporary carriers of atoms or functional groups in the redox and group transfer reactions associated with the metabolism of ingested food in order to obtain energy from the food.
Transamination reaction is a biochemical reaction that involves the transfer of an amino group. In transamination reaction exchange of an amino group from an
A biochemical reaction in which an
A urea cycle is a cyclic biochemical pathway that involves the production of urea using ammonium ions and aspartate molecules as nitrogen sources. The reactants in the formation of carbamoyl phosphate are ammonium ion, water, and carbon dioxide. The desired product of the urea cycle is urea.
There are 20 standard amino acids. Each amino acid has a different carbon skeleton and has a different degradation pathway for its carbon skeleton.
(d)
Answer to Problem 15.116EP
Explanation of Solution
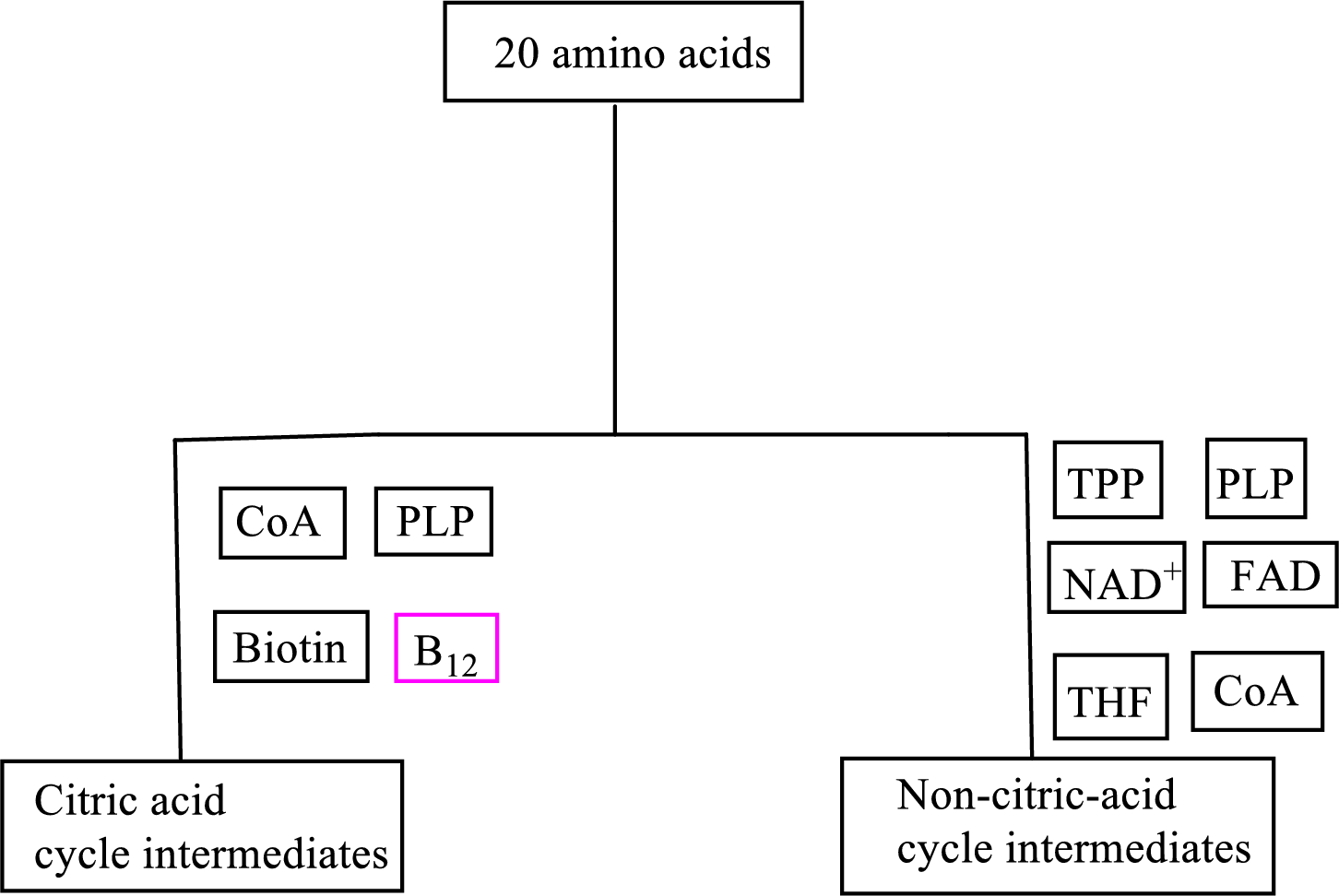
Coenzyme methylcobalamin contains the B vitamin
Coenzyme methylcobalamin is involved in the carbon skeleton degradation pathway to CAC intermediates.
An overview of the B vitamin participations in the degradation pathways for the carbon skeletons of the 20 standard amino acids is as follows:
Want to see more full solutions like this?
Chapter 15 Solutions
Organic And Biological Chemistry
- Which of the following metals is the only one with all of its bands completely full? Group of answer choices K Na Ca Alarrow_forward2. Specify the solvent and reagent(s) required to carry out each of the following FGI. If two reagent sets must be used for the FGI, specify the solvent and reagent(s) for each reagent set. If a reaction cannot be carried out with reagents (sets) class, write NP (not possible) in the solvent box for reagent set #1. Use the letter abbreviation for each solvent; use a number abbreviation for reagent(s). Solvents: CH2Cl2 (A); Reagents: H₂O (B); CH3CO₂H (D) NaHCO3 (4); Hg(OAc)2 (5); HBr (1); R₂BH (6); H2SO4 (2); CH3OH (C); Br₂ (3); H₂O₂ / HO- (7); NaBH4 (8) Reagent Set #1 Reagent Set #2 FGI OH - α-α Br + enant Solvent Reagent(s) Solvent Reagent(s)arrow_forwardBased on concepts from Lecture 3-5, which of the following ionic compounds should be most soluble in water? Group of answer choices MgO BeO CaO BaOarrow_forward
- From an energy standpoint, which two process - in the correct order - are involved in the dissolving of an ionic compound crystal? Group of answer choices Water coordination to the ions followed by sublimation into the gas phase Sublimation of the crystal into gas-phase ions followed by water coordination to the ions Ion dissociation from the crystal followed by water coordination to the ions Water coordination to the ions followed by ion dissociation from the crystalarrow_forwardFor which Group 2 metal (M), is this process the most exothermic? M2+(g) + O2−(g) + CO2(g) → MO(s) + CO2(g) Group of answer choices M = Sr M = Mg M = Ca M = Baarrow_forward2. Specify the solvent and reagent(s) required to carry out each of the following FGI. If two reagent sets must be used for the FGI, specify the solvent and reagent(s) for each reagent set. If a reaction cannot be carried out with reagents (sets) class, write NP (not possible) in the solvent box for reagent set #1. Use the letter abbreviation for each solvent; use a number abbreviation for reagent(s). Solvents: CH2Cl2 (A); H₂O (B); Reagents: HBr (1); H2SO4 (2); CH3OH (C); Br₂ (3); CH3CO₂H (D) NaHCO3 (4); Hg(OAc)2 (5); R₂BH (6); H₂O₂ / HO- (7); NaBH4 (8) Reagent Set #1 Reagent Set #2 FGI Solvent Reagent(s) Solvent Reagent(s) HO OHarrow_forward
- For which of the following ionic compounds would you expect the smallest difference between its theoretical and experimental lattice enthalpies? (You may assume these all have the same unit cell structure.) Electronegativities: Ca (1.0), Fe (1.8), Mg (1.2), O (3.5), S (2.5), Zn (1.6) Group of answer choices ZnO MgS CaO FeSarrow_forwardIn the Born-Haber cycle for KCl crystal formation, what enthalpy component must be divided by two? Group of answer choices KCl(s) enthalpy of formation Ionization energy for K(g) K(s) sublimation enthalpy Cl2 bond dissociation enthalpyarrow_forward2. Specify the solvent and reagent(s) required to carry out each of the following FGI. If two reagent sets must be used for the FGI, specify the solvent and reagent(s) for each reagent set. If a reaction cannot be carried out with reagents (sets) class, write NP (not possible) in the solvent box for reagent set #1. Use the letter abbreviation for each solvent; use a number abbreviation for reagent(s). Solvents: CH2Cl2 (A); H₂O (B); Reagents: HBr (1); R₂BH (6); H2SO4 (2); CH3OH (C); Br₂ (3); CH3CO₂H (D) NaHCO3 (4); Hg(OAc)2 (5); H₂O₂ / HO (7); NaBH4 (8) Reagent Set #1 Reagent Set #2 FGI хот Br Solvent Reagent(s) Solvent Reagent(s)arrow_forward
- What is the correct chemical equation for the lattice formation reaction for CaBr2? Group of answer choices Ca2+(g) + 2 Br−(g) → CaBr2(s) ½ Ca2+(g) + Br−(g) → ½ CaBr2(s) Ca(s) + Br2(l) → CaBr2(s) Ca(s) + 2 Br−(g) → CaBr2(s)arrow_forwardPLEASE ANSWER THE QUESTION!!!arrow_forward3. SYNTHESIS. Propose a sequence of synthetic steps (FGI) that convert the starting material (SM) into the Target molecule. For each FGI in your proposed synthesis, specify the reagents / conditions, and draw the product(s) of that FGI. DO NOT INCLUDE the FGI mxn in the answer you submit. If an FGI requires two reagent sets, specify the order in which the reagent sets are added, e.g., i) Hg(OAc)2 / H₂O; ii) NaBH4/MeOH. Indicate the stereochemistry (if any) of the products of each FGI. FGI 1. Me Starting Material Source of all carbons in the Target molecule (can use multiple copies) Me Me Target molecule + enantiomerarrow_forward
 General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning, Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co




