
Introduction to General, Organic and Biochemistry
11th Edition
ISBN: 9781285869759
Author: Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 14, Problem 14.73P
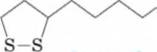 14-73 Lipoic acid is a growth factor for many bacteria
14-73 Lipoic acid is a growth factor for many bacteria
and protozoa and an essential component of several enzymes involved in human
COOH
Lipoic acid
- Name the two
functional groups in lipoic acid.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Could you please turn this into a complete Lewis dot structure formula for me so I can visualize it more clearly? and then do the explaining for the resonance structures that were given please.
Could you please turn this into a complete Lewis dot structure formula for me so I can visualize it more clearly? and then do the explaining for the question.
please solve. If the answer is "no error" and it asks me to type something, and i typed a-helix, its always wrong.
Chapter 14 Solutions
Introduction to General, Organic and Biochemistry
Ch. 14.1 - Prob. 14.1PCh. 14.1 - Prob. 14.2PCh. 14.2 - Problem 14-3 Draw structural formulas for the...Ch. 14.2 - Prob. 14.4PCh. 14.2 - Prob. 14.5PCh. 14.3 - Problem 14-6 Write the common name for each ether.Ch. 14.4 - Prob. 14.7PCh. 14 - 14-8 Answer true or false. The functional group of...Ch. 14 - 14-9 What is the difference in structure between a...Ch. 14 - 14-10 Which of the following are secondary...
Ch. 14 - 14-11 Which of the alcohols in Problem 14-10 are...Ch. 14 - 14-12 Write the 1UPAC name of each compound. (e)...Ch. 14 - Prob. 14.13PCh. 14 - Prob. 14.14PCh. 14 - 14-15 Both alcohols and phenols contain an —OH...Ch. 14 - Prob. 14.16PCh. 14 - 14-17 Explain in terms of noncovalent interactions...Ch. 14 - Prob. 14.18PCh. 14 - Prob. 14.19PCh. 14 - 14-20 Show hydrogen bonding between methanol and...Ch. 14 - 14-21 Show hydrogen bonding between the oxygen of...Ch. 14 - 14-22 Arrange these compounds in order of...Ch. 14 - 14-23 Arrange these compounds in order of...Ch. 14 - 14-24 2-Propanol (isopropyl alcohol) is commonly...Ch. 14 - 14-25 Explain why glycerol is much thicker (more...Ch. 14 - Prob. 14.26PCh. 14 - Prob. 14.27PCh. 14 - 14-28 Give the structural formula of an alkene or...Ch. 14 - Prob. 14.29PCh. 14 - 14-30 Show how to distinguish between cyclohexanol...Ch. 14 - 14-31 Compare the acidity of alcohols and phenols,...Ch. 14 - 14-32 Both 2,6-diisopropylcyclohexanol and the...Ch. 14 - 14-33 Write equations for the reaction of...Ch. 14 - 14-34 Write equations for the reaction of...Ch. 14 - 14-35 Write equations for the reaction of each of...Ch. 14 - 14-36 Show how to convert cyclohexanol to these...Ch. 14 - Prob. 14.37PCh. 14 - Prob. 14.38PCh. 14 - 14-39 Name two important alcohols derived from...Ch. 14 - 14-40 Name two important alcohols derived from...Ch. 14 - Prob. 14.41PCh. 14 - 14-42 Write the common name for each ether. ch3...Ch. 14 - Prob. 14.43PCh. 14 - 14-44 Answer true or false. (a) The functional...Ch. 14 - Prob. 14.45PCh. 14 - Prob. 14.46PCh. 14 - 14-47 Following are structural formulas for...Ch. 14 - 14-48 Explain why methanethiol, CH3SH, has a lower...Ch. 14 - 14-49 Answer true or false. Today, the major...Ch. 14 - Prob. 14.50PCh. 14 - 14-51 (Chemical Connections 14B) When was...Ch. 14 - 14-52 (Chemical Connections 14B) What was Alfred...Ch. 14 - Prob. 14.53PCh. 14 - 14-54 (Chemical Connections 14C) What is the color...Ch. 14 - 14-55 (Chemical Connections 140 The legal...Ch. 14 - 14-56 (Chemical Connections 14D) What does it mean...Ch. 14 - 14-57 (Chemical Connections 14E) What are the...Ch. 14 - Prob. 14.58PCh. 14 - Prob. 14.59PCh. 14 - 14-60 Write a balanced equation for the complete...Ch. 14 - 14-61 Knowing what you do about electronegativity,...Ch. 14 - 14-62 Draw structural formulas and write IUPAC...Ch. 14 - Prob. 14.63PCh. 14 - 14-64 Explain why the boiling point of ethylene...Ch. 14 - Prob. 14.65PCh. 14 - 14-66 1,4-Butanediol, hexane, and 1-pentanol have...Ch. 14 - 14-67 Of the three compounds given in Problem...Ch. 14 - Prob. 14.68PCh. 14 - 14-69 Show how to prepare each compound from...Ch. 14 - 14-70 Show how to prepare each compound from...Ch. 14 - 14-71 The mechanism of the acid-catalyzed...Ch. 14 - Prob. 14.72PCh. 14 - 14-73 Lipoic acid is a growth factor for many...Ch. 14 - 14-74 Following is a structural formula for the...Ch. 14 - Prob. 14.75PCh. 14 - Prob. 14.76PCh. 14 - Prob. 14.77PCh. 14 - 14-78 Consider alkenes A, B, and C. each of which...Ch. 14 - Prob. 14.79P
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Can you please solve and explain this for me in a simple way? I cant seem to comprehend this problem.arrow_forwardPart I. Problem solving. Include all necessary calculations 13 provide plots and graphs. Complexation wl diphenyl carbazide (OPC) in acidic media is another type of sensitive photometric method used for the analysis of aqueous. hexavalent chromium. At 540nm the cherry-red complex as a result of DPC reaction w/ chromium can be photometrically measured. at this wavelength. - a 25mL The UV-vis analysis for the determination of nexavalent chromium in ground water sample is given below. The experiment was based on external calibration method w/ each measurement sample prepared are as follows lab sample analysis contained the standard 100 ppb croy cor groundwater sample, volumes used as indicated below), 12.50 mL of 0.02 M H2Soy and 5.50 ml of 100 ppm DPC (wi water to adjust final volume to 25-ml). The main stripping method was square wave voltammetry, following the conditions set in the main ASV experiment. Standard 100 Volumetric Groundwater H2SO4 0.20 M, flask Sample, mL ppb CrO4*, 100…arrow_forwardplease helparrow_forward
- Predict the products of the following reactions. Draw mechanism arrows for each step for a, b, and c. a.) HBr b.) HI H₂O H2SO4 d.) C12 HO H2SO4 1.) BH3 2.) H2O2, NaOHarrow_forwardK for the following reaction is 0.11 at constant temperature. If the equilibrium concentration of HCl is 0.5 M, what is the equilibrium concentration of NH3. NH4CI(s) ⇌ NH3(g) + HCI(g)arrow_forwardplease help by Draw the following structures (Lewis or line-angle drawing).arrow_forward
- please helparrow_forwardConsider the reaction: 2 A (aq) ⇌ B(aq) Given the following KC values and starting with the initial concentration of A = 4.00 M, complete ICE diagram(s)and find the equilibrium concentrations for A and B.A) KC = 4.00B) KC = 200C) KC = 8.00 x10-3arrow_forward5) Consider the reaction: Cl2 (g) + F2 (g) ⟷ 2 ClF (g) KP=? The partial pressure of 203 kPa for Cl2 and a partial pressure of 405 kPa for F2. Upon reaching equilibrium, thepartial pressure of ClF is 180 kPa. Calculate the equilibrium concentrations and then find the value for KP.arrow_forward
- Don't used hand raiting and don't used Ai solutionarrow_forward(9 Pts) In one of the two Rare Earth element rows of the periodic table, identify an exception tothe general ionization energy (IE) trend. For the two elements involved, answer the followingquestions. Be sure to cite sources for all physical data that you use.a. (2 pts) Identify the two elements and write their electronic configurations.b. (2 pts) Based on their configurations, propose a reason for the IE trend exception.c. (5 pts) Calculate effective nuclear charges for the last electron in each element and theAllred-Rochow electronegativity values for the two elements. Can any of these valuesexplain the IE trend exception? Explain how (not) – include a description of how IErelates to electronegativity.arrow_forwardDon't used hand raiting and don't used Ai solutionarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:9781285869759
Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:Cengage Learning
Acid-Base Titration | Acids, Bases & Alkalis | Chemistry | FuseSchool; Author: FuseSchool - Global Education;https://www.youtube.com/watch?v=yFqx6_Y6c2M;License: Standard YouTube License, CC-BY