
Introduction to General, Organic and Biochemistry
11th Edition
ISBN: 9781285869759
Author: Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Chapter 14, Problem 14.45P
Interpretation Introduction
(a)
Interpretation:
The IUPAC name of the given thiol compound should be determined.
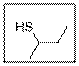
Concept Introduction:
- Firstly, identify the longest chain of carbon atoms and give the name of the chain which is considered as the base name of the compound.
- Numbering of carbon atoms is done in such a way that the
functional group gets the lowest number. - For thiol functional group, “thiol” suffix is used.
Interpretation Introduction
(b)
Interpretation:
The IUPAC name of the given thiol compound should be determined.

Concept Introduction:
- Firstly, identify the longest chain of carbon atoms and give the name of the chain which is considered as the base name of the compound.
- Numbering of carbon atoms is done in such a way that the functional group gets the lowest number.
- For thiol functional group, “thiol” suffix is used.
Interpretation Introduction
(c)
Interpretation:
The IUPAC name of the given thiol compound should be determined.
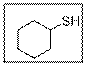
Concept Introduction:
- Firstly, identify the longest chain of carbon atoms and give the name of the chain which is considered as the base name of the compound.
- Numbering of carbon atoms is done in such a way that the functional group gets the lowest number.
- For thiol functional group, “thiol” suffix is used.
Expert Solution & Answer
Trending nowThis is a popular solution!

Students have asked these similar questions
How to draw the mechanism for this reaction??
3) Draw a detailed mechanism and predict the product of the reaction shown?
1) EtMgBr
2) H3O+
How to draw the mechanism for this reaction?
Chapter 14 Solutions
Introduction to General, Organic and Biochemistry
Ch. 14.1 - Prob. 14.1PCh. 14.1 - Prob. 14.2PCh. 14.2 - Problem 14-3 Draw structural formulas for the...Ch. 14.2 - Prob. 14.4PCh. 14.2 - Prob. 14.5PCh. 14.3 - Problem 14-6 Write the common name for each ether.Ch. 14.4 - Prob. 14.7PCh. 14 - 14-8 Answer true or false. The functional group of...Ch. 14 - 14-9 What is the difference in structure between a...Ch. 14 - 14-10 Which of the following are secondary...
Ch. 14 - 14-11 Which of the alcohols in Problem 14-10 are...Ch. 14 - 14-12 Write the 1UPAC name of each compound. (e)...Ch. 14 - Prob. 14.13PCh. 14 - Prob. 14.14PCh. 14 - 14-15 Both alcohols and phenols contain an —OH...Ch. 14 - Prob. 14.16PCh. 14 - 14-17 Explain in terms of noncovalent interactions...Ch. 14 - Prob. 14.18PCh. 14 - Prob. 14.19PCh. 14 - 14-20 Show hydrogen bonding between methanol and...Ch. 14 - 14-21 Show hydrogen bonding between the oxygen of...Ch. 14 - 14-22 Arrange these compounds in order of...Ch. 14 - 14-23 Arrange these compounds in order of...Ch. 14 - 14-24 2-Propanol (isopropyl alcohol) is commonly...Ch. 14 - 14-25 Explain why glycerol is much thicker (more...Ch. 14 - Prob. 14.26PCh. 14 - Prob. 14.27PCh. 14 - 14-28 Give the structural formula of an alkene or...Ch. 14 - Prob. 14.29PCh. 14 - 14-30 Show how to distinguish between cyclohexanol...Ch. 14 - 14-31 Compare the acidity of alcohols and phenols,...Ch. 14 - 14-32 Both 2,6-diisopropylcyclohexanol and the...Ch. 14 - 14-33 Write equations for the reaction of...Ch. 14 - 14-34 Write equations for the reaction of...Ch. 14 - 14-35 Write equations for the reaction of each of...Ch. 14 - 14-36 Show how to convert cyclohexanol to these...Ch. 14 - Prob. 14.37PCh. 14 - Prob. 14.38PCh. 14 - 14-39 Name two important alcohols derived from...Ch. 14 - 14-40 Name two important alcohols derived from...Ch. 14 - Prob. 14.41PCh. 14 - 14-42 Write the common name for each ether. ch3...Ch. 14 - Prob. 14.43PCh. 14 - 14-44 Answer true or false. (a) The functional...Ch. 14 - Prob. 14.45PCh. 14 - Prob. 14.46PCh. 14 - 14-47 Following are structural formulas for...Ch. 14 - 14-48 Explain why methanethiol, CH3SH, has a lower...Ch. 14 - 14-49 Answer true or false. Today, the major...Ch. 14 - Prob. 14.50PCh. 14 - 14-51 (Chemical Connections 14B) When was...Ch. 14 - 14-52 (Chemical Connections 14B) What was Alfred...Ch. 14 - Prob. 14.53PCh. 14 - 14-54 (Chemical Connections 14C) What is the color...Ch. 14 - 14-55 (Chemical Connections 140 The legal...Ch. 14 - 14-56 (Chemical Connections 14D) What does it mean...Ch. 14 - 14-57 (Chemical Connections 14E) What are the...Ch. 14 - Prob. 14.58PCh. 14 - Prob. 14.59PCh. 14 - 14-60 Write a balanced equation for the complete...Ch. 14 - 14-61 Knowing what you do about electronegativity,...Ch. 14 - 14-62 Draw structural formulas and write IUPAC...Ch. 14 - Prob. 14.63PCh. 14 - 14-64 Explain why the boiling point of ethylene...Ch. 14 - Prob. 14.65PCh. 14 - 14-66 1,4-Butanediol, hexane, and 1-pentanol have...Ch. 14 - 14-67 Of the three compounds given in Problem...Ch. 14 - Prob. 14.68PCh. 14 - 14-69 Show how to prepare each compound from...Ch. 14 - 14-70 Show how to prepare each compound from...Ch. 14 - 14-71 The mechanism of the acid-catalyzed...Ch. 14 - Prob. 14.72PCh. 14 - 14-73 Lipoic acid is a growth factor for many...Ch. 14 - 14-74 Following is a structural formula for the...Ch. 14 - Prob. 14.75PCh. 14 - Prob. 14.76PCh. 14 - Prob. 14.77PCh. 14 - 14-78 Consider alkenes A, B, and C. each of which...Ch. 14 - Prob. 14.79P
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- > H₂C=C-CH2-CH3 B. H₂O Pt C. + H2 + H₂O H D. 16. Give the IUPAC name for each of the following: B. Cl Cl c. Cl Cl 17. Draw the line-angle formula for each of the following compounds: 1. phenol 2. 1,3-dichlorobenzene 3. 4-ethyltoluene < Previous Submit Assignment Next ▸arrow_forwardno Ai walkthroughsarrow_forwardThe answer is shown. What is the reaction mechanism to arrive at the answer?arrow_forward
- no Ai walkthroughsarrow_forwardConsider the following nucleophilic substitution reaction. The compound listed above the arrow is the solvent for the reaction. If nothing is listed over the arrow, then the nucleophile is also the solvent for the reaction. Part 1 of 2 Br CH,CN + I¯ What is the correct mechanism for the reaction? Select the single best answer. @SN2 ○ SN 1 Part: 1/2 Part 2 of 2 Draw the products for the reaction. Include both the major organic product and the inorganic product. If more than one stereoisomer is possible, draw only one stereoisomer. Include stereochemistry where relevant. Click and drag to start drawing a structure. X હૈarrow_forward20.33 Think-Pair-Share (a) Rank the following dienes and dienophiles in order of increasing reactivity in the Diels-Alder reaction. (i) CO₂Et (ii) COEt || CO₂Et MeO MeO (b) Draw the product that results from the most reactive diene and most reactive dienophile shown in part (a). (c) Draw a depiction of the orbital overlap involved in the pericyclic reaction that oc- curs between the diene and dienophile in part (b). (d) Is the major product formed in part (b) the endo or exo configuration? Explain your reasoning.arrow_forward
- 20.40 The following compound undergoes an intramolecular Diels-Alder reaction to give a tricyclic product. Propose a structural formula for the product. CN heat An intramolecular Diels-Alder adductarrow_forwardWhat is the reaction mechanism for this? Can this even be done without a base?arrow_forwardWhat is the reaction mechanism for this?arrow_forward
- What is the reaction mechanism for this?arrow_forwardWhat is the reaction mechanism for this?arrow_forwardCurved arrows are used to illustrate the flow of electrons. Using the provided starting and product structures, draw the curved electron-pushing arrows for the following reaction or mechanistic step(s). Be sure to account for all bond-breaking and bond-making steps. + Drawing Arrows CH3ONA, CH3OH heat : Br:O Na → H H Br Na + H H H H H :0: .H + Undo Reset Done Q CH3 Drag To Pan +arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning, General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:9781285869759
Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:Cengage Learning

Organic And Biological Chemistry
Chemistry
ISBN:9781305081079
Author:STOKER, H. Stephen (howard Stephen)
Publisher:Cengage Learning,

General, Organic, and Biological Chemistry
Chemistry
ISBN:9781285853918
Author:H. Stephen Stoker
Publisher:Cengage Learning

Chemistry for Today: General, Organic, and Bioche...
Chemistry
ISBN:9781305960060
Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning