
Concept explainers
(a)
Interpretation:
The predominant organic product that is formed in the given reaction has to be written.
Concept Introduction:
Alcohol is an organic compound that has hydroxyl as its functional group. Alcohols contain both nonpolar and polar groups in it. Hydroxyl group is the polar group and the alkyl group is the nonpolar group. Physical properties of alcohol depend on which of the two groups dominate. Alcohols can be prepared in laboratory by hydration of
Alkenes are compounds that contain a double bond between carbon atoms. When alkenes undergo hydration in presence of sulfuric acid as catalyst, an alcohol is formed as product. The major product formed in case of unsymmetrical alkene is found by using Markovnikov’s rule. The general scheme for hydration of alkene can be given as,
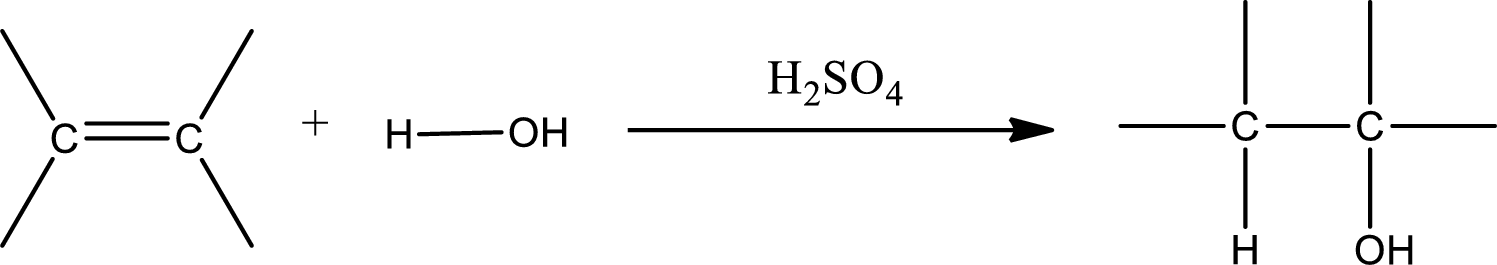
(b)
Interpretation:
The predominant organic product that is formed in the given reaction has to be written.
Concept Introduction:
Alcohol is an organic compound that has hydroxyl as its functional group. Alcohols contain both nonpolar and polar groups in it. Hydroxyl group is the polar group and the alkyl group is the nonpolar group. Physical properties of alcohol depend on which of the two groups dominate. Alcohols can be prepared in laboratory by hydration of alkenes and reduction of carbonyl compounds.
Double bond between a carbon atom and oxygen atom means that the compound is a carbonyl compound. Addition of hydrogen to this carbonyl group leads to the formation of alcohol. When hydrogen is added to the carbonyl, the oxygen of the carbonyl is converted into hydroxyl group. A scheme for the addition of hydrogen to the carbonyl group can be given as shown below,
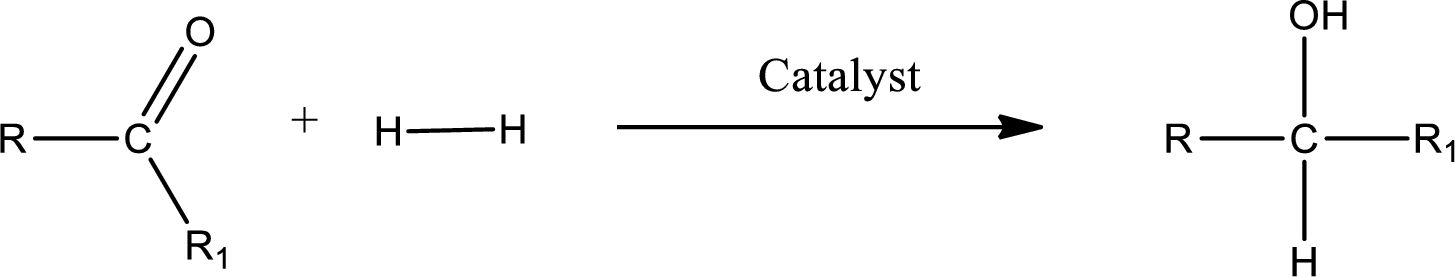
(c)
Interpretation:
The predominant organic product that is formed in the given reaction has to be written.
Concept Introduction:
Alcohol is an organic compound that has hydroxyl as its functional group. Alcohols contain both nonpolar and polar groups in it. Hydroxyl group is the polar group and the alkyl group is the nonpolar group. Physical properties of alcohol depend on which of the two groups dominate. Alcohols can be prepared in laboratory by hydration of alkenes and reduction of carbonyl compounds.
Double bond between a carbon atom and oxygen atom means that the compound is a carbonyl compound. Addition of hydrogen to this carbonyl group leads to the formation of alcohol. When hydrogen is added to the carbonyl, the oxygen of the carbonyl is converted into hydroxyl group. A scheme for the addition of hydrogen to the carbonyl group can be given as shown below,
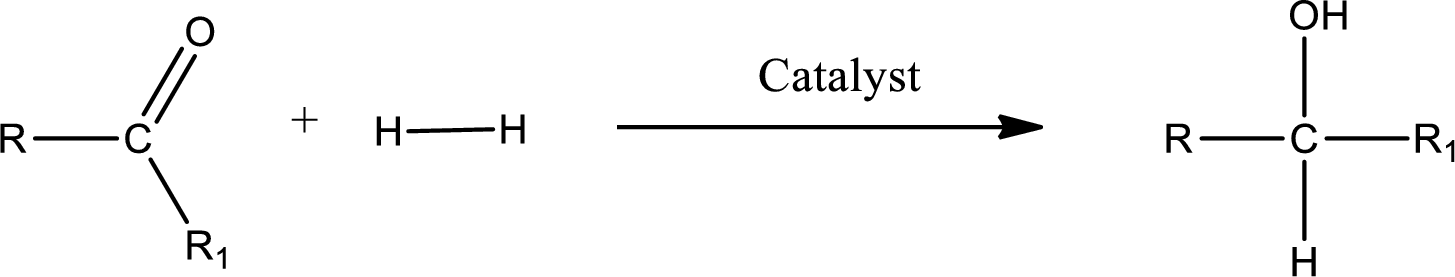
(d)
Interpretation:
The predominant organic product that is formed in the given reaction has to be written.
Concept Introduction:
Alcohol is an organic compound that has hydroxyl as its functional group. Alcohols contain both nonpolar and polar groups in it. Hydroxyl group is the polar group and the alkyl group is the nonpolar group. Physical properties of alcohol depend on which of the two groups dominate. Alcohols can be prepared in laboratory by hydration of alkenes and reduction of carbonyl compounds.
Alkenes are compounds that contain a double bond between carbon atoms. When alkenes undergo hydration in presence of sulfuric acid as catalyst, an alcohol is formed as product. The major product formed in case of unsymmetrical alkene is found by using Markovnikov’s rule. The general scheme for hydration of alkene can be given as,
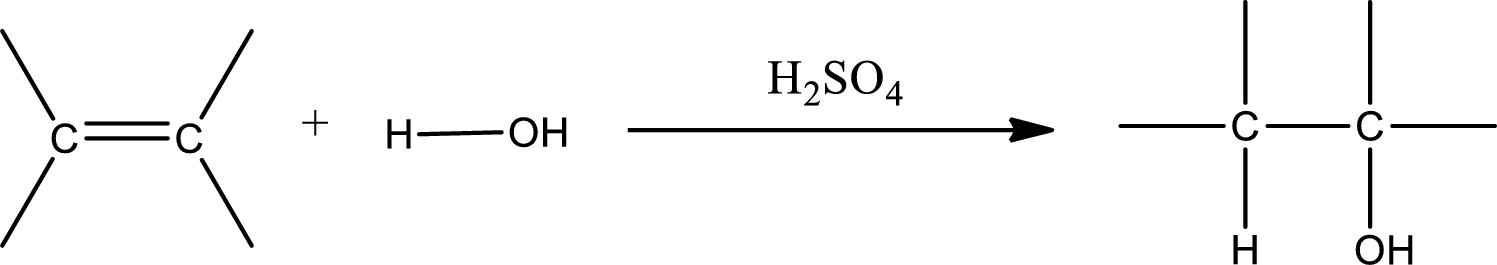
Want to see the full answer?
Check out a sample textbook solution
Chapter 14 Solutions
General, Organic, And Biological Chemistry, Hybrid (with Owlv2 Quick Prep For General Chemistry Printed Access Card)
- For the condensation reaction between Alamine and histamine, please help me write the amididation reaction mechanism. Then write the three letter code for the product of the reaction, then write the one letter code for the product of the reaction. arrow_forwardHow to draw the reaction mechasnism belowarrow_forwardName the following molecules with IUpacarrow_forward
- What is the molecular orbital for cyclopropenyl anion and is it aromatic, antiaromatic or nonaromatic?arrow_forwardUsing the chart describe the change from cystine to tyrosine and its impact on the protein. Using the chart describe the change from histidine to aspartic acid and its impact on the protein.arrow_forwardHow to get the predicted product of this reaction belowarrow_forward
- Please help me fill out the chart then using the chart describe the change from cystine to tyrosine and its impact on the protein. Then using the chart describe the change from histidine to aspartic acid.arrow_forwardWrite the Esterification reaction mechanism for acetic acid, and one propanol to make propanol ethanoate (molecule that gives peas its odor in flavor)arrow_forwardProvide solutionsarrow_forward
 Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning, General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning World of ChemistryChemistryISBN:9780618562763Author:Steven S. ZumdahlPublisher:Houghton Mifflin College Div
World of ChemistryChemistryISBN:9780618562763Author:Steven S. ZumdahlPublisher:Houghton Mifflin College Div





