
Chemistry for Today: General Organic and Biochemistry
9th Edition
ISBN: 9781337514576
Author: Seager
Publisher: Cengage
expand_more
expand_more
format_list_bulleted
Textbook Question
Chapter 14, Problem 14.38E
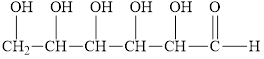
Glucose, the sugar present within the blood, gives a positive Benedict’s test. Circle the structural features that enable glucose to react.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
At 0oC and 1 atm, the viscosity of hydrogen (gas) is 8.55x10-5 P. Calculate the viscosity of a gas, if possible, consisting of deuterium. Assume that the molecular sizes are equal.
Indicate the correct option for the velocity distribution function of gas molecules:a) its velocity cannot be measured in any other way due to the small size of the gas moleculesb) it is only used to describe the velocity of particles if their density is very high.c) it describes the probability that a gas particle has a velocity in a given interval of velocitiesd) it describes other magnitudes, such as pressure, energy, etc., but not the velocity of the molecules
Indicate the correct option for the velocity distribution function of gas molecules:a) its velocity cannot be measured in any other way due to the small size of the gas moleculesb) it is only used to describe the velocity of particles if their density is very high.c) it describes the probability that a gas particle has a velocity in a given interval of velocitiesd) it describes other magnitudes, such as pressure, energy, etc., but not the velocity of the molecules
Chapter 14 Solutions
Chemistry for Today: General Organic and Biochemistry
Ch. 14 - Prob. 14.1ECh. 14 - Prob. 14.2ECh. 14 - Identify each of the following compounds as an...Ch. 14 - Identify each of the following compounds as an...Ch. 14 - Prob. 14.5ECh. 14 - Prob. 14.6ECh. 14 - Prob. 14.7ECh. 14 - Prob. 14.8ECh. 14 - Draw structural formulas and give IUPAC names for...Ch. 14 - Draw structural formulas and give IUPAC names for...
Ch. 14 - Each of the following names is wrong. Give the...Ch. 14 - Each of the following names is wrong. Give the...Ch. 14 - Prob. 14.13ECh. 14 - Prob. 14.14ECh. 14 - Prob. 14.15ECh. 14 - Explain why propane boils at 42C, whereas ethanal,...Ch. 14 - Use a dotted line to show hydrogen bonding between...Ch. 14 - Use a dotted line to show hydrogen bonding between...Ch. 14 - Prob. 14.19ECh. 14 - Prob. 14.20ECh. 14 - Prob. 14.21ECh. 14 - Prob. 14.22ECh. 14 - Prob. 14.23ECh. 14 - Prob. 14.24ECh. 14 - Label each of the following as acetals, ketals, or...Ch. 14 - Label each of the following as acetals, ketals, or...Ch. 14 - Label each of the following structures as a cyclic...Ch. 14 - Label each of the following structures as a...Ch. 14 - What two functional groups react to form the...Ch. 14 - Hemiacetals are sometimes referred to as potential...Ch. 14 - Complete the following statements: a. Oxidation of...Ch. 14 - Prob. 14.32ECh. 14 - Prob. 14.33ECh. 14 - Prob. 14.34ECh. 14 - Prob. 14.35ECh. 14 - Not all aldehyde give a positve Bendicts test....Ch. 14 - A stockroom assistant prepares three bottles, each...Ch. 14 - Glucose, the sugar present within the blood, gives...Ch. 14 - Fructose, present with glucose in honey, reacts...Ch. 14 - Prob. 14.40ECh. 14 - Prob. 14.41ECh. 14 - Complete the following equations. If no reaction...Ch. 14 - Complete the following equations. If no reaction...Ch. 14 - Describe the products that result when hydrogen...Ch. 14 - Prob. 14.45ECh. 14 - Draw structural formulas for the products of the...Ch. 14 - The following compounds are cyclic acetals or...Ch. 14 - The following compounds are cyclic acetals or...Ch. 14 - Write equations to show how the following...Ch. 14 - Prob. 14.50ECh. 14 - Identify the most important aldehyde and ketone...Ch. 14 - Using Table 14.3, name an aldehyde or ketone used...Ch. 14 - Prob. 14.53ECh. 14 - CH3COH(O)CH3COOHacetaldehydeaceticacid You need to...Ch. 14 - The addition of water to aldehydes and ketones...Ch. 14 - Prob. 14.56ECh. 14 - Formaldehyde levels above 0.10mg/1000L of ambient...Ch. 14 - In the IUPAC name for the following ketone, it is...Ch. 14 - Why can formaldehyde (CH2O) be prepared in the...Ch. 14 - Other addition reactions of aldehydes occur....Ch. 14 - Prob. 14.61ECh. 14 - Prob. 14.62ECh. 14 - Vanilla flavoring is either extracted from a...Ch. 14 - Prob. 14.64ECh. 14 - The use of acetone in laboratory experiments must...Ch. 14 - Prob. 14.66ECh. 14 - Prob. 14.67ECh. 14 - Which of the following would be classified as a...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- The number of imaginary replicas of a system of N particlesA) can never become infiniteB) can become infiniteC) cannot be greater than Avogadro's numberD) is always greater than Avogadro's number.arrow_forwardElectronic contribution to the heat capacity at constant volume A) is always zero B) is zero, except for excited levels whose energy is comparable to KT C) equals 3/2 Nk D) equals Nk exp(BE)arrow_forwardPlease correct answer and don't used hand raitingarrow_forward
- Calculate the packing factor of CaTiO3. It has a perovskite structure. Data: ionic radii Co²+ = 0.106 nm, Ti4+ = 0.064 nm, O² = 0.132 nm; lattice constant is a = 2(rTi4+ + ro2-). Ca2+ 02- T14+ Consider the ions as rigid spheres. 1. 0.581 or 58.1% 2. -0.581 or -58.1 % 3. 0.254 or 25.4%arrow_forwardGeneral formula etherarrow_forwardPlease provide the retrosynthetic analysis and forward synthesis of the molecule on the left from the starting material on the right. Please include hand-drawn structures! will upvote! Please correct answer and don't used hand raitingarrow_forward
- Please provide the retrosynthetic analysis and forward synthesis of the molecule on the left from the starting material on the right. Please include hand-drawn structures! will upvote!arrow_forward(please correct answer and don't used hand raiting) Please provide the retrosynthetic analysis and forward synthesis of the molecule on the left from the starting material on the right. Please include hand-drawn structures! will upvote!arrow_forwardCaTiO3 has a perovskite structure. Calculate the packing factor.Data: ionic radii Co+2 = 0.106 nm, Ti+4 = 0.064 nm, O-2 = 0.132 nm; lattice constant is a = 2(rTi4+ + rO-2).(a) 0.581(b) -0.581(c) 0.254(d) -0.254arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY
GCSE Chemistry - Differences Between Compounds, Molecules & Mixtures #3; Author: Cognito;https://www.youtube.com/watch?v=jBDr0mHyc5M;License: Standard YouTube License, CC-BY