
Concept explainers
Draw structural formulas for the products of the following hydrolysis reactions:
a. 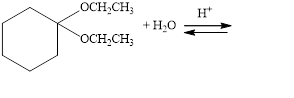
b. 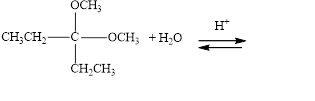
c.
d. 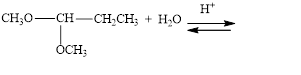
Trending nowThis is a popular solution!

Chapter 14 Solutions
Chemistry for Today: General Organic and Biochemistry
- Draw the curved-arrow mechanism with the drawings of the molecules, not just abbreviations. -NO₂ Sn, HCl (aq) E D H (CH3CO)₂O -NH2 CH3arrow_forwardWhat is/are the product(s) of the following reaction? Select all that apply. * HI A B C OD OH A B OH D Carrow_forwardIn the image, the light blue sphere represents a mole of hydrogen atoms, the purple or teal spheres represent a mole of a conjugate base. A light blue sphere by itself is H+. Assuming there is 2.00 L of solution, answer the following: The Ka of the left & right solution is? The pH of the left & right solution is? The acid on the left & right is what kind of acid?arrow_forward
- What spectral features allow you to differentiate the product from the starting material? Use four separate paragraphs for each set of comparisons. You should have one paragraph each devoted to MS, HNMR, CNMR and IR. 2) For MS, the differing masses of molecular ions are a popular starting point. Including a unique fragmentation is important, too. 3) For HNMR, CNMR and IR state the peaks that are different and what makes them different (usually the presence or absence of certain groups). See if you can find two differences (in each set of IR, HNMR and CNMR spectra) due to the presence or absence of a functional group. Include peak locations. Alternatively, you can state a shift of a peak due to a change near a given functional group. Including peak locations for shifted peaks, as well as what these peaks are due to. Ideally, your focus should be on not just identifying the differences but explaining them in terms of functional group changes.arrow_forwardQuestion 6 What is the major product of the following Diels-Alder reaction? ? Aldy by day of A. H о B. C. D. E. OB OD Oc OE OAarrow_forwardNonearrow_forward
- Chemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
 Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning  Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning





