
Concept explainers
(a)
Interpretation:
HMG-CoA is encountered in the process (1) glycerol
Concept introduction:
Lipogenesis is the process employed for the synthesis of fatty acid. The starting precursor for the synthesis is acetyl CoA. The enzyme employed for the process is fatty acid synthase. It is a multienzyme complex that ties the reaction responsible for the synthesis of fatty acid. The fatty acid is synthesized in two parts. In the first part, there is citrate-malate shuttle system and in the second part, there is a cyclic process to synthesize saturated fatty acid.
The fatty acids are broken down to provide energy. The breakdown of fatty acids is a three parts process. In the first part, the fatty acid is activated. In the second part, the transportation of fatty acid into the mitochondrial matrix is facilitated by a shuttle mechanism. In the third part, the fatty acid is readily oxidized, cycling through a series of four reactions. In these series of reactions, acyl CoA is degraded to acetyl CoA. This pathway is termed as β-oxidation pathway.
Ketogenesis is a metabolic process by which
Triacylglycerol mobilization is an ongoing process in which triacylglycerols that are stored in the adipose tissue are hydrolyzed. Fatty acids and glycerol are the products of triacylglycerol mobilization. The products are released into the bloodstream.
After entering the bloodstream, the glycerol travels to the kidneys or liver. The first stage of glycerol metabolism occurs in the liver or kidney where it is converted to
(a)
Answer to Problem 14.104EP
HMG-CoA is encountered in ketogenesis.
Explanation of Solution
HMG-CoA is produced in step 2 in ketogenesis.
Step 2 is a condensation reaction. In step 2, acetoacetyl CoA reacts with acetyl CoA and water to produce 3-hydroxyl-3-methylglutaryl (HMG-CoA) and CoA-SH. The reaction for step 2 is:
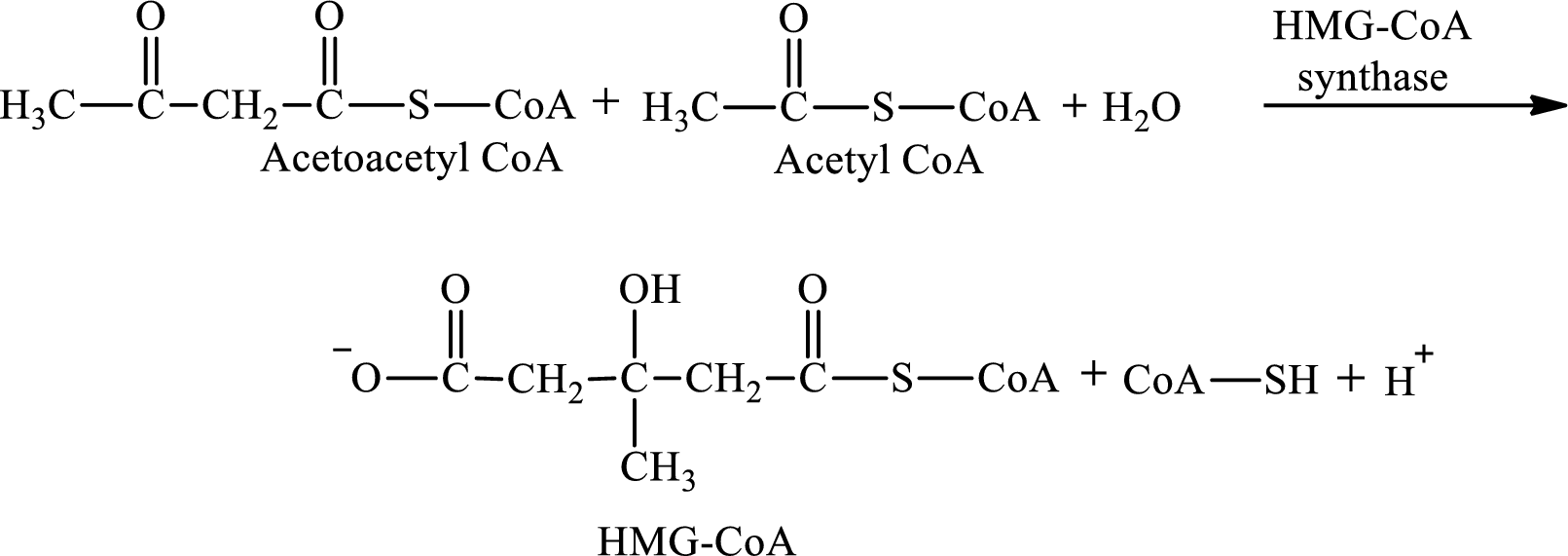
(b)
Interpretation:
NADPH is encountered in the process (1) glycerol metabolism to dihydroxyacetone phosphate, (2) β-oxidation pathway, (3) ketogenesis, and (4) lipogenesis has to be identified.
Concept introduction:
Lipogenesis is the process employed for the synthesis of fatty acid. The starting precursor for the synthesis is acetyl CoA. The enzyme employed for the process is fatty acid synthase. It is a multienzyme complex that ties the reaction responsible for the synthesis of fatty acid. The fatty acid is synthesized in two parts. In the first part, there is citrate-malate shuttle system and in the second part, there is a cyclic process to synthesize saturated fatty acid.
The fatty acids are broken down to provide energy. The breakdown of fatty acids is a three parts process. In the first part, the fatty acid is activated. In the second part, the transportation of fatty acid into the mitochondrial matrix is facilitated by a shuttle mechanism. In the third part, the fatty acid is readily oxidized, cycling through a series of four reactions. In these series of reactions, acyl CoA is degraded to acetyl CoA. This pathway is termed as β-oxidation pathway.
Ketogenesis is a metabolic process by which ketone bodies are produced by the breakdown of fatty acids and ketogenic amino acids. This metabolic process supplies our organs with needed energy under certain circumstances such as starvation. Fatty acid molecules degrade into acetyl CoA which are utilized as reactants in the process of ketogenesis. These molecules of acetyl CoA undergo the process of condensation twice, followed by chain cleavage and hydrogenation to produce ketone bodies.
Triacylglycerol mobilization is an ongoing process in which triacylglycerols that are stored in the adipose tissue are hydrolyzed. Fatty acids and glycerol are the products of triacylglycerol mobilization. The products are released into the bloodstream.
After entering the bloodstream, the glycerol travels to the kidneys or liver. The first stage of glycerol metabolism occurs in the liver or kidney where it is converted to
(b)
Answer to Problem 14.104EP
NADPH is encountered in lipogenesis.
Explanation of Solution
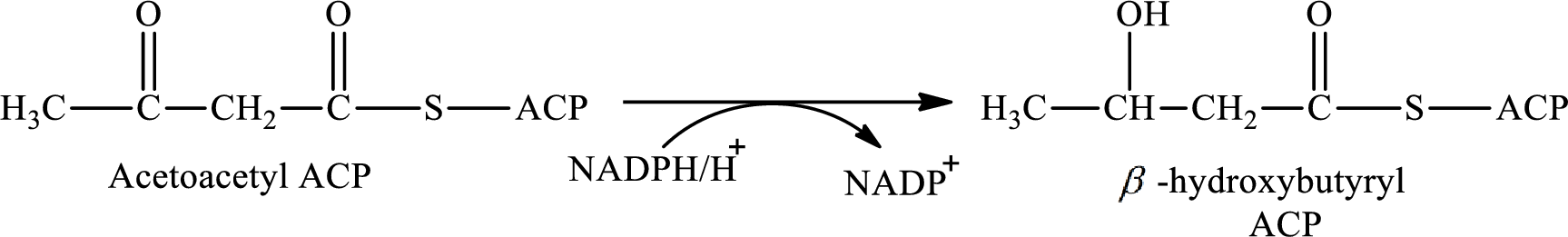
NADPH acts as the reducing agent in step 2 and 4 of the cyclic process of lipogenesis. NADPH gets oxidized to form NADP+.
Step 2 involves the hydrogenation of acetoacetyl ACP to synthesis β-hydroxybutyrylwith the help of reducing agent NADPH. The reaction of this step is:
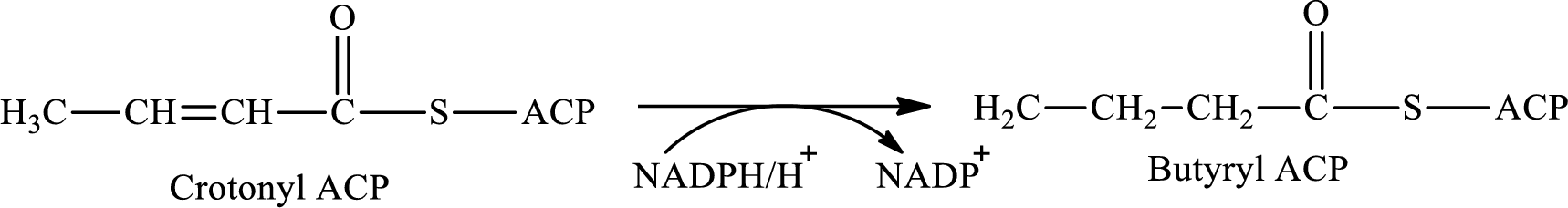
Step 4 again involves hydrogenation reaction. In this step, crotonyl ACP is converted to butyryl ACP with the help of reducing agent NADPH. The reaction of this step is:
(c)
Interpretation:
Malonyl ACP is encountered in the process (1) glycerol metabolism to dihydroxyacetone phosphate, (2) β-oxidation pathway, (3) ketogenesis, and (4) lipogenesis has to be identified.
Concept introduction:
Lipogenesis is the process employed for the synthesis of fatty acid. The starting precursor for the synthesis is acetyl CoA. The enzyme employed for the process is fatty acid synthase. It is a multienzyme complex that ties the reaction responsible for the synthesis of fatty acid. The fatty acid is synthesized in two parts. In the first part, there is citrate-malate shuttle system and in the second part, there is a cyclic process to synthesize saturated fatty acid.
The fatty acids are broken down to provide energy. The breakdown of fatty acids is a three parts process. In the first part, the fatty acid is activated. In the second part, the transportation of fatty acid into the mitochondrial matrix is facilitated by a shuttle mechanism. In the third part, the fatty acid is readily oxidized, cycling through a series of four reactions. In these series of reactions, acyl CoA is degraded to acetyl CoA. This pathway is termed as β-oxidation pathway.
Ketogenesis is a metabolic process by which ketone bodies are produced by the breakdown of fatty acids and ketogenic amino acids. This metabolic process supplies our organs with needed energy under certain circumstances such as starvation. Fatty acid molecules degrade into acetyl CoA which are utilized as reactants in the process of ketogenesis. These molecules of acetyl CoA undergo the process of condensation twice, followed by chain cleavage and hydrogenation to produce ketone bodies.
Triacylglycerol mobilization is an ongoing process in which triacylglycerols that are stored in the adipose tissue are hydrolyzed. Fatty acids and glycerol are the products of triacylglycerol mobilization. The products are released into the bloodstream.
After entering the bloodstream, the glycerol travels to the kidneys or liver. The first stage of glycerol metabolism occurs in the liver or kidney where it is converted to
(c)
Answer to Problem 14.104EP
Malonyl ACP is encountered in lipogenesis.
Explanation of Solution
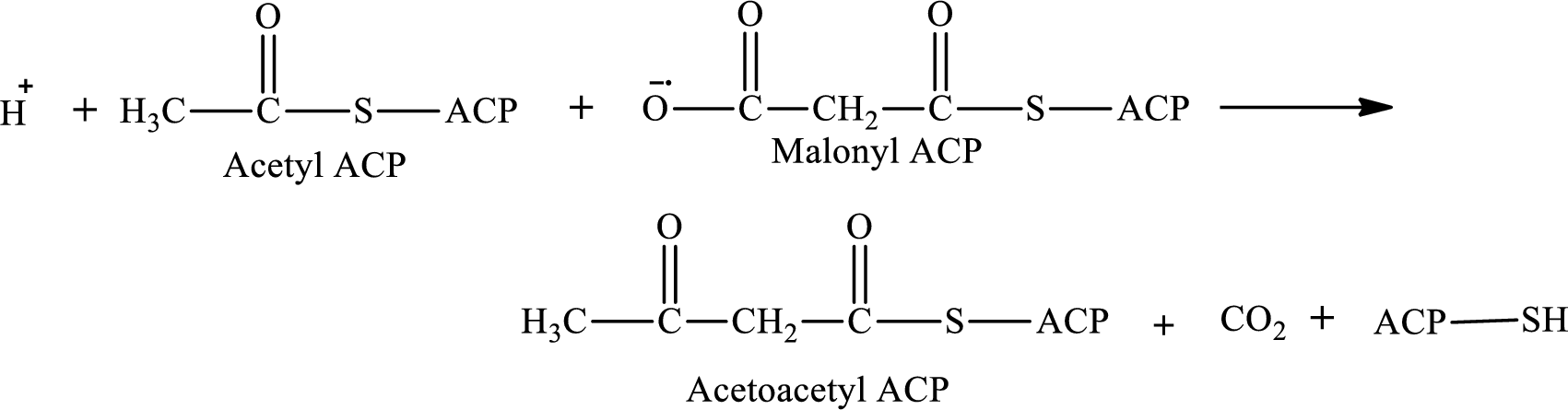
Malonyl ACP is the reactant in step 1 in the cyclic process in lipogenesis.
The first step involves the condensation reaction between acetyl ACP and malonyl ACP. The product formed in the first reaction is acetoacetyl ACP. The reaction of step 1 is:
(d)
Interpretation:
Acetoacetyl CoA is encountered in the process (1) glycerol metabolism to dihydroxyacetone phosphate, (2) β-oxidation pathway, (3) ketogenesis, and (4) lipogenesis has to be identified.
Concept introduction:
Lipogenesis is the process employed for the synthesis of fatty acid. The starting precursor for the synthesis is acetyl CoA. The enzyme employed for the process is fatty acid synthase. It is a multienzyme complex that ties the reaction responsible for the synthesis of fatty acid. The fatty acid is synthesized in two parts. In the first part, there is citrate-malate shuttle system and in the second part, there is a cyclic process to synthesize saturated fatty acid.
The fatty acids are broken down to provide energy. The breakdown of fatty acids is a three parts process. In the first part, the fatty acid is activated. In the second part, the transportation of fatty acid into the mitochondrial matrix is facilitated by a shuttle mechanism. In the third part, the fatty acid is readily oxidized, cycling through a series of four reactions. In these series of reactions, acyl CoA is degraded to acetyl CoA. This pathway is termed as β-oxidation pathway.
Ketogenesis is a metabolic process by which ketone bodies are produced by the breakdown of fatty acids and ketogenic amino acids. This metabolic process supplies our organs with needed energy under certain circumstances such as starvation. Fatty acid molecules degrade into acetyl CoA which are utilized as reactants in the process of ketogenesis. These molecules of acetyl CoA undergo the process of condensation twice, followed by chain cleavage and hydrogenation to produce ketone bodies.
Triacylglycerol mobilization is an ongoing process in which triacylglycerols that are stored in the adipose tissue are hydrolyzed. Fatty acids and glycerol are the products of triacylglycerol mobilization. The products are released into the bloodstream.
After entering the bloodstream, the glycerol travels to the kidneys or liver. The first stage of glycerol metabolism occurs in the liver or kidney where it is converted to
(d)
Answer to Problem 14.104EP
Acetoacetyl CoA is encountered in ketogenesis.
Explanation of Solution
Step 2 is a condensation reaction. In step 2, acetoacetyl CoA reacts with acetyl CoA and water to produce 3-hydroxyl-3-methylglutaryl (HMG-CoA) and CoA-SH. The reaction for step 2 is:
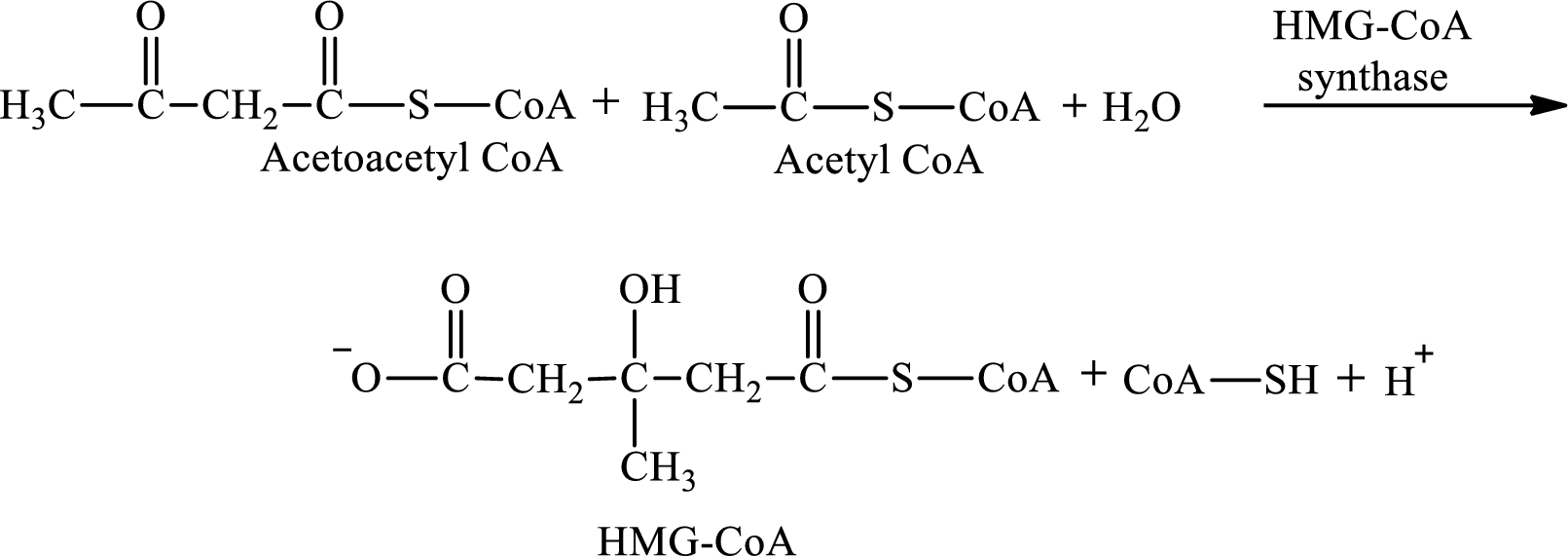
Want to see more full solutions like this?
Chapter 14 Solutions
EBK ORGANIC AND BIOLOGICAL CHEMISTRY
- NAME: 1. Draw the major product of the following E2 reaction. Make sure you pay attention to REGIOCHEMISTRY and STEREOCHEMISTRY. To get credit for this question, you must EXPLAIN how you got your answer using STRUCTURES and WORDS. Br NaOCH3 acetone F2 reaction To get credit for thisarrow_forward3. Reactions! Fill in the information missing below. Make sure to pay attention to REGIOCHEMISTRY and STEREOCHEMISTRY. Br2 CH3OH + 4. Mechanism! Show the complete arrow pushing mechanism, including all steps and intermediates for the following reactions. To get credit for this, you MUST show how ALL bonds are broken and formed, using arrows to show the movement of electrons. H3O+ HOarrow_forwardPlease provide a synthesis for the Ester using proponoic acid, thank you!arrow_forward
- Please help with the curved arrow mechanism of this reaction, thank youarrow_forwardConcentration (mg/l) Peak Area 0 158 10 10241 20 18425 30 26457 40 37125 50 44256 60 56124 Question: Determine the regression equation (a and b coefficients) from first principlesarrow_forwardConcentration (mg/l) Peak Area 0 158 10 10241 20 18425 30 26457 40 37125 50 44256 60 56124 You have been asked to determine the concentration of citral in a highly valued magnolia essential oil. QUESTION: Calculate the concentration of citral in your highly valued magnolia essential oil which returns a peak area of 41658arrow_forward
- Need help with these problems...if you can please help me understand problems E & F.arrow_forwardPlease help me solve these problems. Thank you in advance.arrow_forwardPredict the products of this organic reaction: O N IN A N + H2O + HCI ? Specifically, in the drawing area below draw the skeletal ("line") structure of the product, or products, of this reaction. If there's more than one product, draw them in any arrangement you like, so long as they aren't touching. If there aren't any products because this reaction won't happen, check the No reaction box under the drawing area. 田 C + Explanation Check Click and drag to start drawing a structure. C © 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Centerarrow_forward
- 6. For each of the following, fill in the synthesis arrows with reagents and show the intermediates. You DO NOT need to use the same number of arrows that are shown (you may use more or less), but the product must be formed from the reactant. Then write the mechanism of one step in the synthesis (you can choose which step to write the mechanism for), including all reagents required, clearly labeling the nucleophile and electrophile for each step, and using curved arrows to show the steps in the mechanism. a. b. OHarrow_forwardDraw the productsarrow_forwardDraw the correct productsarrow_forward
 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning, Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning



