
Interpretation:
The mechanism for the given reaction between 2,3-dimethyl-1,3-butadiene and hydrogen bromide is to be determined. The reaction energy coordinate diagram that illustrates kinetic and thermodynamic pathways is to be drawn.
Concept introduction:
舧 Electrophiles are electron-deficient species, which has positive or partially positive charge. Lewis acids are electrophiles, which accept electron pair.
舧 Nucleophiles are electron-rich species, which has negative or partially negative charge. Lewis bases are nucleophiles, which donate electron pair.
舧 Free radical is an atom, molecule, or ion that has an unpaired electron, which makes it highly chemically reactive.
舧 Substitution reaction: A reaction in which one of the hydrogen atoms of a hydrocarbon or a
舧 Elimination reaction: A reaction in which two substituent groups are detached and a double bond is formed is called elimination reaction.
舧 Addition reaction: It is the reaction in which unsaturated bonds are converted to saturated molecules by the addition of molecules.
舧 The reaction in which there is addition of hydrogen molecule is called hydrogenation reaction.
舧
舧 Hydrogenation with platinum as a catalyst is used to convert unsaturated carbohydrates to saturated hydrocarbons
舧 Oxidation of
舧 Ozonolysis helps convert the carbon – carbon double bonds to carbon – oxygen double bond (carbonyl compounds).
舧 Dimethyl sulfide is used as a reducing agent that decomposes the intermediate formed into the carbonyl group.
舧 NBS (nitro-bromo succinimide) is a special reagent used for bromination of allylic carbocations.
舧 Bromine replaces the hydrogen attached to the carbon adjacent to the carbon bearing double bond.
舧 This method of using NBS can produce allylic bromides without bromine reacting with the double bond.
舧 Dehydration of a primary alcohol in the presence of a mineral acid like concentrated sulfuric acid results in the formation of alkene via E2 elimination.
舧 1,2–addition of hydrohalogenation (HX) to a diene is the addition of hydrogen to the carbon designated as 1 and halogen to the carbon designated as 2. The positions of carbon as 1 and 2 are not according to the IUPAC numbering of the molecule, but as a conjugated diene. The mechanism is similar to 1,4-addition. The mechanism of 1,2-addition and 1,4-addition of hydrohalogenation is given below:
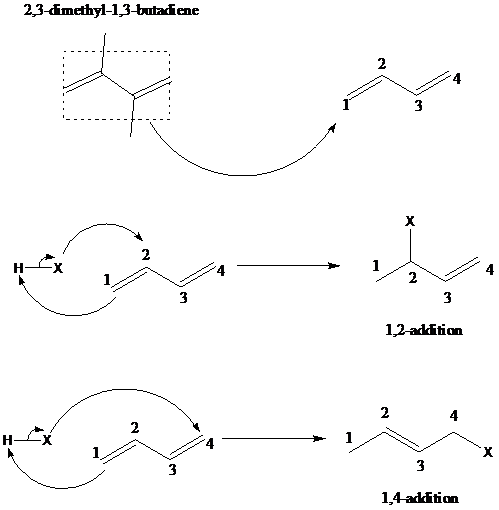
舧 When conjugated diene is attacked by an electrophile, the resultant products are formed via 1,2-addition and 1,4-addition reaction mechanism. Kinetics and
Want to see the full answer?
Check out a sample textbook solution
Chapter 13 Solutions
Organic Chemistry
- Identify and provide an explanation of the operational principles behind a Atomic Absorption Spectrometer (AAS). List the steps involved.arrow_forwardInstructions: Complete the questions in the space provided. Show all your work 1. You are trying to determine the rate law expression for a reaction that you are completing at 25°C. You measure the initial reaction rate and the starting concentrations of the reactions for 4 trials. BrO³¯ (aq) + 5Br¯ (aq) + 6H* (aq) → 3Br₂ (l) + 3H2O (l) Initial rate Trial [BrO3] [H*] [Br] (mol/L) (mol/L) | (mol/L) (mol/L.s) 1 0.10 0.10 0.10 8.0 2 0.20 0.10 0.10 16 3 0.10 0.20 0.10 16 4 0.10 0.10 0.20 32 a. Based on the above data what is the rate law expression? b. Solve for the value of k (make sure to include proper units) 2. The proposed reaction mechanism is as follows: i. ii. BrО¸¯ (aq) + H+ (aq) → HBrO3 (aq) HBrO³ (aq) + H* (aq) → H₂BrO3* (aq) iii. H₂BrO³* (aq) + Br¯ (aq) → Br₂O₂ (aq) + H2O (l) [Fast] [Medium] [Slow] iv. Br₂O₂ (aq) + 4H*(aq) + 4Br(aq) → 3Br₂ (l) + H2O (l) [Fast] Evaluate the validity of this proposed reaction. Justify your answer.arrow_forwardе. Д CH3 D*, D20arrow_forward
- H3C. H3C CH 3 CH 3 CH3 1. LDA 2. PhSeCl 3. H2O2arrow_forwardPlease predict the products for each of the following reactions: 1.03 2. H₂O NaNH, 1. n-BuLi 2. Mel A H₂ 10 9 0 H2SO4, H₂O HgSO4 Pd or Pt (catalyst) B 9 2 n-BuLi ♡ D2 (deuterium) Lindlar's Catalyst 1. NaNH2 2. EtBr Na, ND3 (deuterium) 2. H₂O2, NaOH 1. (Sia)2BH с Darrow_forwardin the scope of ontario SCH4U grade 12 course, please show ALL workarrow_forward
- Is the chemical reaction CuCl42-(green) + 4H2O <==> Cu(H2O)42+(blue) + 4Cl- exothermic or endothermic?arrow_forwardIf we react tetraethoxypropane with hydrazine, what is the product obtained (explain its formula). State the reason why the corresponding dialdehyde is not used.arrow_forwarddrawing, no aiarrow_forward

 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning


