
Organic Chemistry
11th Edition
ISBN: 9781118133576
Author: T. W. Graham Solomons, Craig Fryhle
Publisher: Wiley, John & Sons, Incorporated
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 13, Problem 4PP
Practice Problem 13.4
From each set of resonance structures that follow, designate the one that would contribute most to the hybrid and explain your choice:
(a)
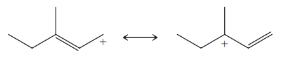
(b)
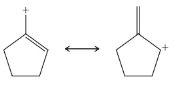
(c)
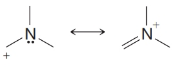
(d)
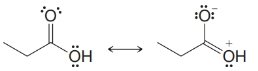
(e)

(f)

Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
What is the product of the reaction of XeF4 with H2O?
Group of answer choices
H2XeF2
H2XeF4
XeO3
H2XeO
While noble gas exerts the strongest London (dispersion) forces on neighboring atoms?
Group of answer choices
Xe
Ar
Kr
Ne
Which of the following elements is corrosive to your skin due to that element breaking down C=C bonds?
Group of answer choices
fluorine
iodine
bromine
chlorine
Chapter 13 Solutions
Organic Chemistry
Ch. 13 - Prob. 1PPCh. 13 - Prob. 2PPCh. 13 - Prob. 3PPCh. 13 - Practice Problem 13.4 From each set of resonance...Ch. 13 - Practice Problem 13.5 The following enol (an...Ch. 13 - Prob. 6PPCh. 13 - Practice Problem 13.7
Two compounds, A and B, have...Ch. 13 - Prob. 8PPCh. 13 - Prob. 9PPCh. 13 - Prob. 10PP
Ch. 13 - Prob. 11PPCh. 13 - Prob. 12PPCh. 13 - Prob. 13PPCh. 13 - Prob. 14PPCh. 13 - Prob. 15PPCh. 13 - Practice Problem 13.16
Diels–Alder reactions also...Ch. 13 - Prob. 17PPCh. 13 - Prob. 18PCh. 13 - What product would you expect from the following...Ch. 13 - Prob. 20PCh. 13 - Prob. 21PCh. 13 - Provide the reagents necessary for each of the...Ch. 13 - Prob. 23PCh. 13 - Prob. 24PCh. 13 - Prob. 25PCh. 13 - When 1-pentene reacts with N-bromosuccinimide...Ch. 13 - Prob. 27PCh. 13 - Prob. 28PCh. 13 - Prob. 29PCh. 13 - Prob. 30PCh. 13 - 13.31 Provide a mechanism that explains formation...Ch. 13 - 13.32 Provide a mechanism that explains formation...Ch. 13 - Treating either 1-chloro-3-methyl-2-butene or...Ch. 13 - Prob. 34PCh. 13 - Prob. 35PCh. 13 - Although both 1-bromobutane and 4-bromo-1-butene...Ch. 13 - Prob. 37PCh. 13 - Prob. 38PCh. 13 - Prob. 39PCh. 13 - Prob. 40PCh. 13 - Prob. 41PCh. 13 - Prob. 42PCh. 13 - Prob. 43PCh. 13 - Prob. 44PCh. 13 - 13.44 When furan and maleimide undergo a...Ch. 13 - Two controversial hard insecticides are aldrin and...Ch. 13 - Prob. 47PCh. 13 - Prob. 48PCh. 13 - Prob. 49PCh. 13 - Prob. 50PCh. 13 - Explain the product distribution below based on...Ch. 13 - Mixing furan (Problem 13.44) with maleic anhydride...Ch. 13 - Prob. 53PCh. 13 - Prob. 54PCh. 13 - Prob. 1LGPCh. 13 - Prob. 2LGPCh. 13 - Prob. 1QCh. 13 - Prob. 2QCh. 13 - Prob. 3QCh. 13 - Prob. 4QCh. 13 - Prob. 5Q
Additional Science Textbook Solutions
Find more solutions based on key concepts
In mechanism, photophosphorylation is most similar to A. substrate-level phosphorylation in glycolysis. B. oxid...
Campbell Biology in Focus (2nd Edition)
The following data were obtained from a disk-diffusion test. Antibiotic Zone of Inhibition A 15 mm B 0 mm c 7 m...
Microbiology: An Introduction
30. Drosophila has a diploid chromosome number of 2n = 8, which includes one pair of sex chromosomes (XX in fem...
Genetic Analysis: An Integrated Approach (3rd Edition)
47. A 250.0-mL buffer solution is 0.250 M in acetic acid and 0.250 M in sodium acetate.
a. What is the initial ...
Chemistry: A Molecular Approach (4th Edition)
2. Define equilibrium population. Outline the conditions that must be met for a population to stay in genetic e...
Biology: Life on Earth (11th Edition)
Describe the various layering arrangements and cell shapes of epithelial tissue.
Principles of Anatomy and Physiology
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- What the best source of sulfide to use on a small scale in the lab? Group of answer choices thiourea H2S NaHS Na2Sarrow_forwardWhich of the following statements about sulfur is FALSE? Group of answer choices H2S is the product of an oxygen-depleted ecosystem. In the acid mine drainage reaction, FeS2 is a product. One allotrope of sulfur has the formula S20. In the environment, bacterial oxidation can convert S2− to elemental S or SO42−.arrow_forwardOf the following choices, which is the best reason that most materials DON'T spontaneously combust even though our atmosphere is about 21% oxygen? Group of answer choices The reduction of O2 in the gas phase (O2 + e− → O2−) is spontaneous. The reduction of O2 in acid solution (O2 + H+ + e− → HO2(aq)) is spontaneous. O2 is not a reactant in combustion. The O2 bond dissociation energy is 494 kJ/mol, leading to a high activation energy for combustion.arrow_forward
- please answer in the scope of the SCH4U course, I am having a hard time understanding, may you show all steps please and thank you! can you also put the final answers in the table so its understandablearrow_forwardPlan the synthesis of the following compound using the starting material provided and any other reagents needed as long as carbon based reagents have 3 carbons or less. Either the retrosynthesis or the forward synthesis (mechanisms are not required but will be graded if provided) will be accepted if all necessary reagents and intermediates are shown (solvents and temperature requirements are not needed unless specifically involved in the reaction, i.e. DMSO in the Swem oxidation or heat in the KMnO4 oxidation). There may be more than one correct answer, and chemically correct steps will be accepted. Extra points will be given if correct names are provided. The points earned here will be applied to your lowest exam score! H Harrow_forwardDraw the mechanism to make the alcohol 1-hexanol. Please use arrows.arrow_forward
- Answer the followings: 1-What is the difference(s) between DNA and RNA: a- Structure: b- Function: c- Types: 2-What is the meaning of: a- Replication b- Transcription c- Translation 3- Show the base pair connection (hydrogen bond) in DNA and RNAarrow_forwardWhy does the anhydride react with the OH on the benzene rather than the OH on the carboxy group?arrow_forwardAnswer the followings: 1- What is the IP for a amino acid? Give example. 2- What are the types of amino acids? 3- What are the structures of protein? 4- The N-Terminal analysis by the Edman method shows saralasin contains sarcosine at the N- terminus. Partial hydrolysis of saralasin with dilute hydrochloric acid yields the following fragments: Tyr-Val-His Sar-Arg-Val His-Pro-Ala Val-Tyr-Val Arg-Val-Tyr What is the structure of saralasin? 5. MATCH a term from the list below to each definition. Place the letter of the term in the blank to the left of the definition. a. Ligases b. Fibrous proteins c. Conjugated protein d. Hydrolases a. b. C. e. Simple protein f. Globular proteins g. Lyases h. Transferases Proteins that are tough and insoluble in water. Enzymes that catalyze the breaking away of a small molecule such as from a substrate. Enzymes that catalyze the bonding together of two substrates.arrow_forward
- Answer the followings (Four): 1-What is the difference(s) between FOUR: a. Glyceride and phosphoglyceride. b. Wax and fat. c. Soap and fatty acid. d. HDL and LDL cholesterol e. Phospho lipids and sphingosine. 2-What are the types of lipids? 3-What are the main lipid components of membrane structures? 4-How could lipids play important rules as signaling molecules and building units? 5. The Structure variety of Lipids makes them to play significant rules in our body. Conclude briefly on this statement.arrow_forwardHO IV но. = HO но. HO. HO но. зад надо What is the product of the following reaction?arrow_forwardDraw the mechanism to make the alcohol 2-hexanol.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you

NMR Spectroscopy; Author: Professor Dave Explains;https://www.youtube.com/watch?v=SBir5wUS3Bo;License: Standard YouTube License, CC-BY