
(a)
Interpretation:
The electron-pair geometry for each carbon atom in
Concept introduction:
The electron pairs in Lewis diagrams repel each other in real molecule and thus they distribute themselves in positions around the central atoms which are far away from one another. This arrangement of electron pairs is called electron-pair geometry. The electron pairs may be shared in covalent bond, or they may be lone pairs.
Answer to Problem 24E
The Lewis diagram for the molecule
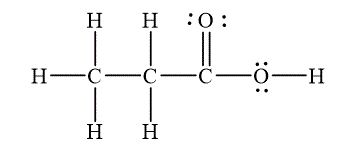
The electron pair geometry for the carbon atoms
Explanation of Solution
To write the Lewis diagram for a compound, first the number of valence electrons is to be calculated. In the molecule,
The atom which is least electronegative and lesser in number is the central atom. In
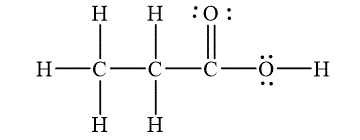
Figure 1
The electron-pair geometry depends on the number of electron pairs around the central atoms. In the molecule
The Lewis diagram for the molecule
(b)
Interpretation:
The molecular geometry predicted by the valence shell electron-pair repulsion theory for each carbon atom in the molecule
Concept introduction:
Molecular geometry is the precise term that is used to describe the shape of molecules and arrangement of atoms around the central atom. The molecular geometry of a molecule is predicted by valence shell electron-pair repulsion theory or in short VSEPR theory. VSEPR theory applies to substances in which a second period element is bonded to two, three, four, or other atoms.
Answer to Problem 24E
The Lewis diagrams for
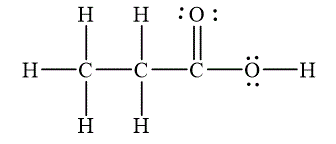
The molecular geometry is tetrahedral for the carbons
Explanation of Solution
To write the Lewis diagram for a compound first the number of valence electrons is to be calculated. In the molecule
The atom which is least electronegative and lesser in number is the central atom. In

Figure 1
The molecular geometry depends on the number of electron pairs as well as number of unpaired electron on the central atoms. In the molecule
The Lewis diagram for the molecule
Want to see more full solutions like this?
Chapter 13 Solutions
Introductory Chemistry: An Active Learning Approach
- Complete the clean-pushing mechanism for the given ether synthesia from propanol in concentrated sulfurica140°C by adding any mining aloms, bands, charges, nonbonding electron pairs, and curved arrows. Draw hydrogen bonded to cayan, when applicable. ore 11,0 HPC Step 1: Draw curved arrows Step 2: Complete the intend carved Q2Q 56 QQQ Step 3: Complete the intermediate and add curved Step 4: Modify the structures to draw the QQQ QQQarrow_forward6. In an experiment the following replicate set of volume measurements (cm3) was recorded: (25.35, 25.80, 25.28, 25.50, 25.45, 25.43) A. Calculate the mean of the raw data. B. Using the rejection quotient (Q-test) reject any questionable results. C. Recalculate the mean and compare it with the value obtained in 2(a).arrow_forwardA student proposes the transformation below in one step of an organic synthesis. There may be one or more reactants missing from the left-hand side, but there are no products missing from the right-hand side. There may also be catalysts, small inorganic reagents, and other important reaction conditions missing from the arrow. • Is the student's transformation possible? If not, check the box under the drawing area. • If the student's transformation is possible, then complete the reaction by adding any missing reactants to the left-hand side, and adding required catalysts, inorganic reagents, or other important reaction conditions above and below the arrow. • You do not need to balance the reaction, but be sure every important organic reactant or product is shown. + T G OH де OH This transformation can't be done in one step.arrow_forward
- Macmillan Leaming Draw the major organic product of the reaction. 1. CH3CH2MgBr 2. H+ - G Select Draw Templates More H о QQarrow_forwardDraw the condensed structure of 3-hydroxy-2-butanone. Click anywhere to draw the first atom of your structure.arrow_forwardGive the expected major product of reaction of 2,2-dimethylcyclopropane with each of the following reagents. 2. Reaction with dilute H₂SO, in methanol. Select Draw Templates More CHC Erase QQQ c. Reaction with dilute aqueous HBr. Select Drew Templates More Era c QQQ b. Reaction with NaOCH, in methanol. Select Draw Templates More d. Reaction with concentrated HBr. Select Draw Templates More En a QQQ e. Reaction with CH, Mg1, then H*, H₂O 1. Reaction with CH,Li, then H', H₂Oarrow_forward
- Write the systematic name of each organic molecule: structure O OH OH name X ☐arrow_forwardMacmillan Learning One of the molecules shown can be made using the Williamson ether synthesis. Identify the ether and draw the starting materials. А со C Strategy: Review the reagents, mechanism and steps of the Williamson ether synthesis. Determine which of the molecules can be made using the steps. Then analyze the two possible disconnection strategies and deduce the starting materials. Identify the superior route. Step 6: Put it all together. Complete the two-step synthesis by selecting the reagents and starting materials. C 1. 2. Answer Bank NaH NaOH NaOCH, снен, сен, он Сиси, Сне (СН), СОН (Сн, Свarrow_forwardWrite the systematic name of each organic molecule: structure CH3 O CH3-CH-CH-C-CH3 OH HV. CH3-C-CH-CH2-CH3 OH CH3 O HO—CH, CH–CH—C CH3 OH 오-오 name X G ☐arrow_forward
- HI Organic Functional Groups Predicting the reactants or products of esterification What is the missing reactant in this organic reaction? HO OH H +回 + H₂O 60013 Naomi V Specifically, in the drawing area below draw the skeletal ("line") structure of R. If there is more than one reasonable answer, you can draw any one of them. If there is no reasonable answer, check the No answer box under the drawing area. No answer Click and drag to start drawing a structure. Explanation Check 1 2 #3 $ 4 2025 % ala5 'a :☐ G & 67 8 Ar K enter Accessible 9 Q W E R TY U 1 tab , S H J Karrow_forwardPlease help me with number 5 using my data and graph. I think I might have number 3 and 4 but if possible please check me. Thanks in advance!arrow_forwarddict the major products of this organic reaction. C Explanation Check 90 + 1.0₂ 3 2. (CH3)2S Click and drag f drawing a stru © 2025 McGraw Hill LLC. All Rights Reserved. • 22 4 5 7 8 Y W E R S F H Bilarrow_forward
 Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
 Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning





