
Fundamentals of General, Organic, and Biological Chemistry (8th Edition)
8th Edition
ISBN: 9780134015187
Author: John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. Peterson
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
thumb_up100%
Chapter 12.2, Problem 12.1P
Locate and identify the
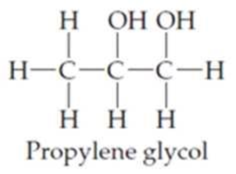
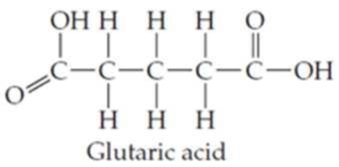
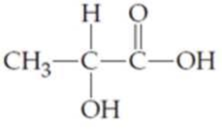
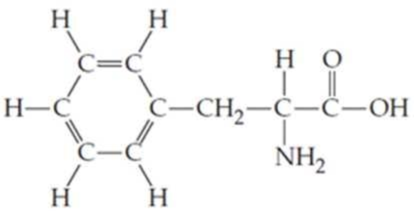
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
12. When glutamic acid is heated to 180°C., it loses a molecule of water to form a
lactam. The bond that creates the lactam is an amide bond that forms between the
amino nitrogen and the carbon of the side chain -COOH group. What is the most
reasonable structure for this lactam? (NOTE: The structure of glutamic acid is shown
in Question 1.)
COOH
COOH
ا کچھ جو کچھ
C
D
COOH
B
COOH
E
COOH
10. Which one of the
following compounds is
the major organic product
of the series of reactions
shown here?
Ph
A
OH
Ph
B
CO₂Et
Br
-H
Ν ΚΑ
CO₂Et
1. NaOEt
1. NaOH, H₂O
2. H3O+
2. PhCH2CH2Br
3. heat
NH2
Ph
OH
NH2
0
OH
Ph
OH
NH2
Ph
D
NH2
E
OH
1. What is the isoelectric point of glutamic acid?
(Glutamic acid has pKa1 2.10, pKa2 4.07, pKaз 9.47)
A) pH 2.1
D) pH 6.8
B) pH 3.1
C) pH 4.1
E) pH 9.5
HO
NH2
Glutamic acid
(shown without charges)
OH
Chapter 12 Solutions
Fundamentals of General, Organic, and Biological Chemistry (8th Edition)
Ch. 12.2 - Locate and identify the functional groups in (a)...Ch. 12.2 - Draw structures for molecules that fit the...Ch. 12.3 - Prob. 12.3PCh. 12.3 - There are two branched-chain isomers with the...Ch. 12.4 - Draw the following three isomers of C5H12 as...Ch. 12.4 - Prob. 12.6PCh. 12.4 - Prob. 12.7PCh. 12.4 - Draw both condensed and line structures for the...Ch. 12.5 - Which of the following structures represent the...Ch. 12.5 - Are the pairs of compounds shown below the same...
Ch. 12.6 - Identify each carbon in the molecule shown in...Ch. 12.6 - Prob. 12.12PCh. 12.6 - Prob. 12.13PCh. 12.6 - Draw and name alkanes that meet the following...Ch. 12.6 - Prob. 12.15KCPCh. 12.6 - Prob. 12.1CIAPCh. 12.6 - Prob. 12.2CIAPCh. 12.6 - Prob. 12.3CIAPCh. 12.8 - Prob. 12.1MRPCh. 12.8 - Prob. 12.2MRPCh. 12.8 - Prob. 12.16PCh. 12.8 - Write the structures of all singly chlorinated...Ch. 12.10 - Prob. 12.18PCh. 12.10 - Prob. 12.19PCh. 12.10 - What is wrong with the following names? It will be...Ch. 12.10 - Prob. 12.21KCPCh. 12.10 - Prob. 12.4CIAPCh. 12.10 - (a) What common produce items might you see...Ch. 12 - Convert the following models into line drawings...Ch. 12 - Prob. 12.23UKCCh. 12 - Prob. 12.24UKCCh. 12 - Give the IUPAC names for the following...Ch. 12 - Prob. 12.26UKCCh. 12 - What characteristics of carbon make possible the...Ch. 12 - Prob. 12.28APCh. 12 - Prob. 12.29APCh. 12 - Prob. 12.30APCh. 12 - For each of the following, give an example of a...Ch. 12 - Identify the highlighted functional groups in the...Ch. 12 - Identify the functional groups in the following...Ch. 12 - Propose structures for molecules that fit the...Ch. 12 - Prob. 12.35APCh. 12 - What requirement must be met for two compounds to...Ch. 12 - Prob. 12.37APCh. 12 - Prob. 12.38APCh. 12 - Prob. 12.39APCh. 12 - Prob. 12.40APCh. 12 - Give an example of a compound that meets the...Ch. 12 - (a)There are two isomers with the formula C4H10....Ch. 12 - Write condensed structures for the following...Ch. 12 - Prob. 12.44APCh. 12 - Prob. 12.45APCh. 12 - Which of the following pairs of structures are...Ch. 12 - Prob. 12.47APCh. 12 - Prob. 12.48APCh. 12 - Prob. 12.49APCh. 12 - What are the IUPAC names of the following alkanes?Ch. 12 - Prob. 12.51APCh. 12 - Write condensed structures for the following...Ch. 12 - Draw line structures for the following...Ch. 12 - Name the following cycloalkanes:Ch. 12 - Prob. 12.55APCh. 12 - Prob. 12.56APCh. 12 - Prob. 12.57APCh. 12 - Prob. 12.58APCh. 12 - Prob. 12.59APCh. 12 - Prob. 12.60APCh. 12 - Prob. 12.61APCh. 12 - Write the formulas of the four singly chlorinated...Ch. 12 - Write the formulas of the three doubly brominated...Ch. 12 - Identify the indicated functional groups in the...Ch. 12 - The line structure for pregabalin (Lyrica) is...Ch. 12 - Prob. 12.66CPCh. 12 - Prob. 12.67CPCh. 12 - Most lipsticks are about 70% castor oil and wax....Ch. 12 - Prob. 12.69CPCh. 12 - Prob. 12.70CPCh. 12 - Prob. 12.71CPCh. 12 - Which of the following structures represent the...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, biochemistry and related others by exploring similar questions and additional content below.Similar questions
- Br Mg, ether 1. HCHO (formaldehyde) 2. H+, H₂O PCC 1. NH3, HCN ? (pyridinium chlorochromate) 2. H2O, HCI 11. Which one of the following compounds is the major organic product of the series of reactions shown above? Ph. Ph. OH NH2₂ A Ph. Ή NH2 B OH Ph Η Ph OH NH2 NH2₂ NH₂ C D Earrow_forwardB A 6. Which ONE of the labeled bonds in the tripeptide on the right is a peptide bond: H₂N N 'N' OH C H A, B, C, D or E? HN E OHarrow_forwardQuestions 8-9 are 0.4 points each. The next two questions relate to the peptide whose structure is shown here. To answer these questions, you should look at a table of H2N/.. amino acid structures. You don't have to memorize the structures of the amino acids. IZ 8. What is the N-terminal amino acid of this peptide? A) proline B) aspartic acid C) threonine 9. What is the C-terminal amino acid of this peptide? A) proline B) aspartic acid C) threonine N OH D) valine E) leucine D) valine E) leucine NH "OH OHarrow_forward
- 7. What is the correct name of the following tripeptide? A) Ile-Met-Ser B) Leu-Cys-Thr C) Val-Cys-Ser D) Ser-Cys-Leu E) Leu-Cys-Ser H₂N!!!!! N H ΖΙ .SH SF H IN OH OHarrow_forwardPlease draw out the following metabolic pathways: (Metabolic Map) Mitochondrion: TCA Cycle & GNG, Electron Transport, ATP Synthase, Lipolysis, Shuttle Systems Cytoplasm: Glycolysis & GNG, PPP (Pentose Phosphate Pathway), Glycogen, Lipogenesis, Transporters and Amino Acids Control: Cori/ Glc-Ala cycles, Insulin/Glucagon Reg, Local/Long Distance Regulation, Pools Used Correctlyarrow_forwardPlease help provide me an insight of what to draw for the following metabolic pathways: (Metabolic Map) Mitochondrion: TCA Cycle & GNG, Electron Transport, ATP Synthase, Lipolysis, Shuttle Systems Cytoplasm: Glycolysis & GNG, PPP (Pentose Phosphate Pathway), Glycogen, Lipogenesis, Transporters and Amino Acids Control: Cori/ Glc-Ala cycles, Insulin/Glucagon Reg, Local/Long Distance Regulation, Pools Used Correctlyarrow_forward
- f. The genetic code is given below, along with a short strand of template DNA. Write the protein segment that would form from this DNA. 5'-A-T-G-G-C-T-A-G-G-T-A-A-C-C-T-G-C-A-T-T-A-G-3' Table 4.5 The genetic code First Position Second Position (5' end) U C A G Third Position (3' end) Phe Ser Tyr Cys U Phe Ser Tyr Cys Leu Ser Stop Stop Leu Ser Stop Trp UCAG Leu Pro His Arg His Arg C Leu Pro Gln Arg Pro Leu Gin Arg Pro Leu Ser Asn Thr lle Ser Asn Thr lle Arg A Thr Lys UCAG UCAC G lle Arg Thr Lys Met Gly Asp Ala Val Gly Asp Ala Val Gly G Glu Ala UCAC Val Gly Glu Ala Val Note: This table identifies the amino acid encoded by each triplet. For example, the codon 5'-AUG-3' on mRNA specifies methionine, whereas CAU specifies histidine. UAA, UAG, and UGA are termination signals. AUG is part of the initiation signal, in addition to coding for internal methionine residues. Table 4.5 Biochemistry, Seventh Edition 2012 W. H. Freeman and Company B eviation: does it play abbreviation:arrow_forwardAnswer all of the questions please draw structures for major productarrow_forwardfor glycolysis and the citric acid cycle below, show where ATP, NADH and FADH are used or formed. Show on the diagram the points where at least three other metabolic pathways intersect with these two.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Human Heredity: Principles and Issues (MindTap Co...BiologyISBN:9781305251052Author:Michael CummingsPublisher:Cengage Learning
Human Heredity: Principles and Issues (MindTap Co...BiologyISBN:9781305251052Author:Michael CummingsPublisher:Cengage Learning Biology 2eBiologyISBN:9781947172517Author:Matthew Douglas, Jung Choi, Mary Ann ClarkPublisher:OpenStax
Biology 2eBiologyISBN:9781947172517Author:Matthew Douglas, Jung Choi, Mary Ann ClarkPublisher:OpenStax Biology Today and Tomorrow without Physiology (Mi...BiologyISBN:9781305117396Author:Cecie Starr, Christine Evers, Lisa StarrPublisher:Cengage Learning
Biology Today and Tomorrow without Physiology (Mi...BiologyISBN:9781305117396Author:Cecie Starr, Christine Evers, Lisa StarrPublisher:Cengage Learning
 Anatomy & PhysiologyBiologyISBN:9781938168130Author:Kelly A. Young, James A. Wise, Peter DeSaix, Dean H. Kruse, Brandon Poe, Eddie Johnson, Jody E. Johnson, Oksana Korol, J. Gordon Betts, Mark WomblePublisher:OpenStax College
Anatomy & PhysiologyBiologyISBN:9781938168130Author:Kelly A. Young, James A. Wise, Peter DeSaix, Dean H. Kruse, Brandon Poe, Eddie Johnson, Jody E. Johnson, Oksana Korol, J. Gordon Betts, Mark WomblePublisher:OpenStax College

Human Heredity: Principles and Issues (MindTap Co...
Biology
ISBN:9781305251052
Author:Michael Cummings
Publisher:Cengage Learning

Biology 2e
Biology
ISBN:9781947172517
Author:Matthew Douglas, Jung Choi, Mary Ann Clark
Publisher:OpenStax

Biology Today and Tomorrow without Physiology (Mi...
Biology
ISBN:9781305117396
Author:Cecie Starr, Christine Evers, Lisa Starr
Publisher:Cengage Learning



Anatomy & Physiology
Biology
ISBN:9781938168130
Author:Kelly A. Young, James A. Wise, Peter DeSaix, Dean H. Kruse, Brandon Poe, Eddie Johnson, Jody E. Johnson, Oksana Korol, J. Gordon Betts, Mark Womble
Publisher:OpenStax College
Metabolic Pathways; Author: Wisc-Online;https://www.youtube.com/watch?v=m61bQYio9ys;License: Standard Youtube License