
Principles of General, Organic, Biological Chemistry
2nd Edition
ISBN: 9780073511191
Author: Janice Gorzynski Smith Dr.
Publisher: McGraw-Hill Education
expand_more
expand_more
format_list_bulleted
Textbook Question
Chapter 12.1, Problem 12.1P
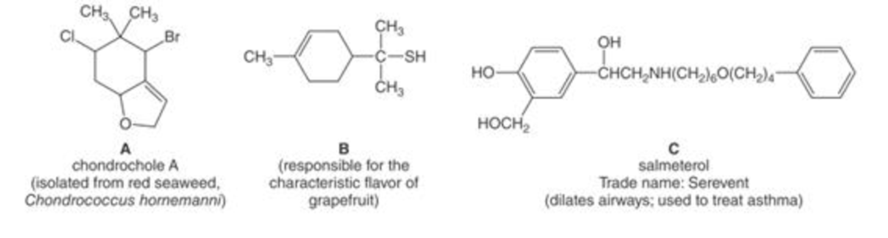
- a. Label the hydroxyl groups, thiols, halogens, and ether oxygens in each compound.
- b. Which –OH group in salmeterol (C) is not part of an alcohol? Explain.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Glycerol contains:
a. oxygens which are each bonded to two alkyl groups
b. oxygens single-bonded to primary and secondary carbons
c. Oxygens double-bonded to carbon, with alkyls on both sides
d. Oxygens double-bonded to carbon, with alkyls on one side only
e. Oxygens double-bonded to carbon, with an alkyl on one side and an --OH on the other side
1. Consider the following common organic molecules and answer the questions below (A-J)
by writing the number of the molecule(s) next to the question.
CH3
CH3
CH3
H;C"
CH2
2. muscone
3. nicotine
1. limonene
H;CCH3
CH3
CH3
H;C,
CH3
0-CH3
H-O
ČO,H
4. ibuprofen
5. camphor
6. vanillin
ÇH3
HO,
H;C
HO.
но-
H
H;C
H3C"
CH3
7. aspirin
8. geraniol
9. acetaminophen
ALCOHOLS
1. WHY IS ETHANOL MORE SOLUBLE IN WATER THAN 1-HEXANOL?
2. WHAT IS DENATURED ALCOHOL? AND WHY IS ALCOHOL DENATURED?
ETHER
1. WHY DOES DIETHYL ETHER HAVE MUCH LOWER BOILING POINT THAN 1-BUTANOL?
Chapter 12 Solutions
Principles of General, Organic, Biological Chemistry
Ch. 12.1 - a. Label the hydroxyl groups, thiols, halogens,...Ch. 12.1 - Draw out each compound to clearly show what groups...Ch. 12.2 - Classify each alcohol as 1, 2, or 3.Ch. 12.2 - Classify each hydroxyl group in sorbitol as 1, 2,...Ch. 12.2 - Which compound in each pair has the higher boiling...Ch. 12.2 - Label each compound as water soluble or water...Ch. 12.2 - Give the IUPAC name for each compound.Ch. 12.2 - Give the structure corresponding to each name. a....Ch. 12.3 - Name each ether. a. CH3OCH2CH2CH2CH3 b....Ch. 12.3 - Prob. 12.10P
Ch. 12.3 - Which compound in each pair has the higher boiling...Ch. 12.5 - Prob. 12.12PCh. 12.5 - Prob. 12.13PCh. 12.6 - Prob. 12.14PCh. 12.6 - Prob. 12.15PCh. 12.6 - Give the structure corresponding to each name. a....Ch. 12.7 - Prob. 12.17PCh. 12.8 - Give the IUPAC name for each aldehyde. a....Ch. 12.8 - Prob. 12.19PCh. 12.8 - Give the IUPAC name for each aldehyde depicted in...Ch. 12.8 - Prob. 12.21PCh. 12.8 - Prob. 12.22PCh. 12.8 - Acetone and progesterone are two ketones that...Ch. 12.9 - Prob. 12.24PCh. 12.10 - Prob. 12.25PCh. 12.11 - Prob. 12.26PCh. 12.11 - Prob. 12.27PCh. 12.11 - Prob. 12.28PCh. 12.11 - Prob. 12.29PCh. 12.11 - Prob. 12.30PCh. 12.11 - Prob. 12.31PCh. 12.11 - Prob. 12.32PCh. 12 - Prob. 12.33UKCCh. 12 - Prob. 12.34UKCCh. 12 - Consider the following ball-and-stick model of an...Ch. 12 - Consider the following ball-and-stick model. a....Ch. 12 - Name each compound. a. CH3CH2OCH2CH2CH2CH3Ch. 12 - Name each compound. a. CH3OCH2CH2CH3 b....Ch. 12 - Answer the following questions about alcohol A. a....Ch. 12 - Answer the following questions about alcohol B. a....Ch. 12 - Prob. 12.41UKCCh. 12 - Prob. 12.42UKCCh. 12 - Prob. 12.43UKCCh. 12 - Prob. 12.44UKCCh. 12 - Prob. 12.45APCh. 12 - Prob. 12.46APCh. 12 - Prob. 12.47APCh. 12 - Prob. 12.48APCh. 12 - Prob. 12.49APCh. 12 - Prob. 12.50APCh. 12 - Prob. 12.51APCh. 12 - Prob. 12.52APCh. 12 - Prob. 12.53APCh. 12 - Give the structure corresponding to each name. a....Ch. 12 - Prob. 12.55APCh. 12 - Draw structures for the four constitutional...Ch. 12 - Prob. 12.57APCh. 12 - Rank the following compounds in order of...Ch. 12 - Explain why two four-carbon organic molecules have...Ch. 12 - Explain why the boiling point of CH3CH2CH2CH2OH...Ch. 12 - Which compound in each pair has the higher boiling...Ch. 12 - Which compound in each pair is more water soluble?...Ch. 12 - Prob. 12.63APCh. 12 - Prob. 12.64APCh. 12 - Prob. 12.65APCh. 12 - Prob. 12.66APCh. 12 - Prob. 12.67APCh. 12 - Xylitol is a nontoxic compound as sweet as table...Ch. 12 - Prob. 12.69APCh. 12 - Prob. 12.70APCh. 12 - Prob. 12.71APCh. 12 - Prob. 12.72APCh. 12 - Prob. 12.73APCh. 12 - Prob. 12.74APCh. 12 - Prob. 12.75APCh. 12 - Prob. 12.76APCh. 12 - Prob. 12.77APCh. 12 - Draw the structure corresponding to each name. a....Ch. 12 - Prob. 12.79APCh. 12 - Prob. 12.80APCh. 12 - What product is formed when each compound is...Ch. 12 - Prob. 12.82APCh. 12 - Prob. 12.83APCh. 12 - Prob. 12.84APCh. 12 - Prob. 12.85APCh. 12 - Prob. 12.86APCh. 12 - Prob. 12.87APCh. 12 - Label each of the following objects as chiral or...Ch. 12 - Prob. 12.89APCh. 12 - Prob. 12.90APCh. 12 - Prob. 12.91APCh. 12 - Prob. 12.92APCh. 12 - Prob. 12.93APCh. 12 - Prob. 12.94APCh. 12 - Prob. 12.95APCh. 12 - Prob. 12.96APCh. 12 - Prob. 12.97APCh. 12 - How are the compounds in each pair related? Are...Ch. 12 - Prob. 12.99APCh. 12 - Prob. 12.100APCh. 12 - Prob. 12.101APCh. 12 - Prob. 12.102APCh. 12 - Prob. 12.103APCh. 12 - Lactic acid [CH3CH(OH)CO2H] gives sour milk its...Ch. 12 - Prob. 12.105APCh. 12 - Prob. 12.106APCh. 12 - Prob. 12.107CPCh. 12 - Prob. 12.108CPCh. 12 - Prob. 12.109BTCCh. 12 - Prob. 12.110BTC
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Draw the structure corresponding to each name. a. propyl formate b. butyl butanoate c. heptyl benzoate d. N-ethylhexanamide e. N-ethyl- N-methylheptanamidearrow_forwardWhich among these have the highest boiling point? Rank them from A-D. Methanol, water, ethanol, and diethyl ether. Please explain thoroughly including each of their intermolecular forces and how it makes them stronger or weaker than the others.arrow_forwardGive the IUPAC name for the following compound. OH Select one: O A. 3-vinyl-1-butanol O B. 3-methyl-4-penten-1-ol O C. 3-methyl-1-pentenyl alcoholarrow_forward
- Draw the structure of a hydrocarbon that has six carbon atoms and a. three vinylic hydrogens and two allylic hydrogens. b. three vinylic hydrogens and one allylic hydrogen. c. three vinylic hydrogens and no allylic hydrogens.arrow_forward1. Give the skeletal structure and the IUPAC name for: a. n-propyl propenyl ether b. isobutyl tert-butyl ether C. sec-butyl alcohol d. cyclohexyl ethyl ether e. neopentyl alcohol f. cyclobutyl phenolarrow_forwardPlease help me solve this problem.arrow_forward
- 1. Simple ketones, like acetone, are often used as industrial solvents for many organically based products such as adhesives and paints. They are considered "universal solvents," because they dissolve so many diverse materials. Explain why these chemicals are good solvents.arrow_forward2. In the past ether was used as an anaesthetic but has been replaced by safer compounds because it is very volatile and flammable. Explain why ether has a lower boiling point than alcohols but higher than alkanes. Please answer this question with the full explanation, its very urgent!arrow_forwardare they: secondary, primary, tertiary or phenol alcohol?arrow_forward
- I. Write the IUPAC name given the structure. .CO2H 'N'arrow_forwardLabel each functional group as an alcohol, ether, acetal, or hemiacetal. LOCH3 a. OCH3 (select) OH b. CH3-C-OCH2CH,CH3 CH3 (select) с. `OCH,CH,CH3 (select) d. OCH,CH,CH3 (select) (select) two ethers hemiacetal acetal alcohol and ether alcohol etherarrow_forwardExplain Ethers & its bonded group ?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning

Chemistry for Today: General, Organic, and Bioche...
Chemistry
ISBN:9781305960060
Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning

Introductory Chemistry: An Active Learning Approa...
Chemistry
ISBN:9781305079250
Author:Mark S. Cracolice, Ed Peters
Publisher:Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Cengage Learning
07 Physical Properties of Organic Compounds; Author: Mindset;https://www.youtube.com/watch?v=UjlSgwq4w6U;License: Standard YouTube License, CC-BY