
Answer the following questions about alcohol A.
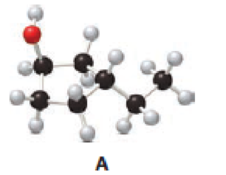
- a. Give the IUPAC name.
- b. Classify the alcohol as 1°, 2°, or 3°.
- c. Draw the products formed when A is dehydrated with H2SO4.
- d. What product is formed when A is oxidized with K2Cr2O7?
- e. Draw a constitutional isomer of A that contains an OH group.
- f. Draw a constitutional isomer of A that contains an ether.
a.
Interpretation:
For the below compound IUPAC name has to be determined.
Concept introduction:
Nomenclature of alcohol:
Firstly, find the longest carbon chain, which is bonded with the
Explanation of Solution
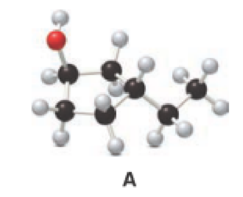
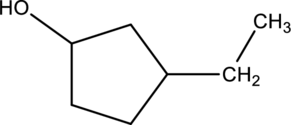
Given compound A is,
Step 1: In the given compound, black, white and red color balls indicates carbon, hydrogen and oxygen atoms respectively. Firstly, the ring which, is attached to the
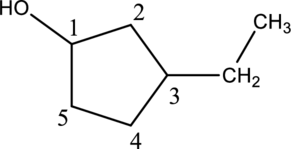
Step 2: For naming the compound, firstly, the parent carbon chain should be checked, and as it possess five membered ring, it is named as cyclopentane. Due to
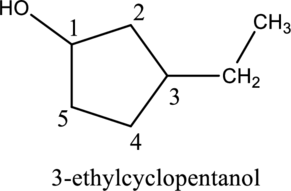
b.
Interpretation:
From the compound A, the type of alcohol has to be determined.
Concept introduction:
Classification of alcohol:
Generally, the alcohol group is bonded with minimum one alkyl group. The alcohol group is classified into three types, such as primary
Explanation of Solution
In the below compound A,
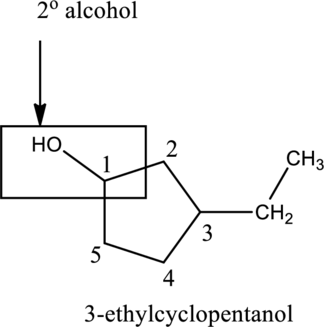
c.
Interpretation:
The product obtained when the compound A reacts with H2SO4 reagent has to be determined.
Concept introduction:
Dehydration of alcohol:
When alcohol reacts with
Explanation of Solution
Given stating material A is 3-ethylcyclopentanol
When 3-ethylcyclopentanol reacts with
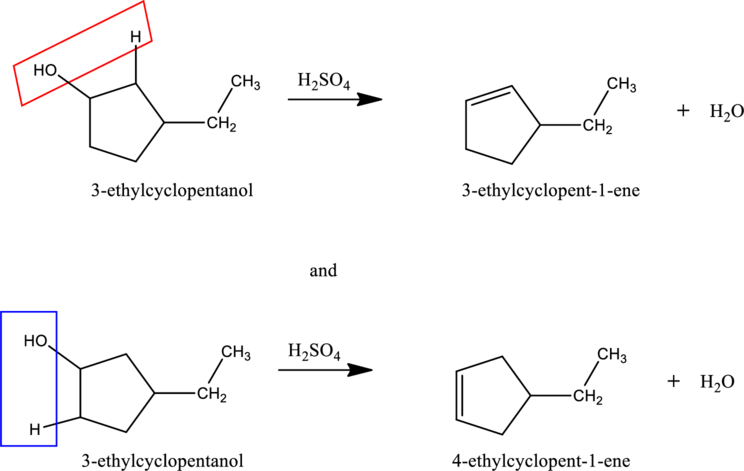
d.
Interpretation:
The product obtained when the compound A reacts with K2Cr2O7 reagent has to be determined.
Concept introduction:
Oxidation of alcohol:
During oxidation of alcohol, the number of
Explanation of Solution
Given stating material A is 3-ethylcyclopentanol
When 3-ethylcyclopentanol, the secondary alcohol containing one hydrogen atom on the carbon attached with OH group reacts with K2Cr2O7, it gets oxidized to 3-ethylcyclopentanone as shown below.
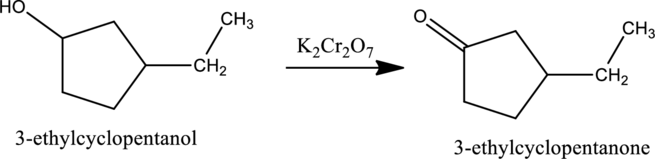
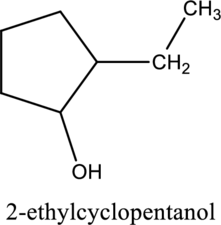
e.
Interpretation:
The constitutional isomer of compound A that contain
Concept introduction:
Constitutional isomers:
The compounds, that contain same molecular formula but different with respect to molecular orientation. In other words compounds having similar molecular formula and different structure are called as Constitutional isomers.
Explanation of Solution
In the given compound A molecular formula is
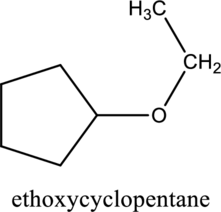
f.
Interpretation:
The constitutional isomer of compound A that contain an ether group has to be determined.
Concept introduction:
Refer to part ‘e.’.
Explanation of Solution
In the given compound A molecular formula is
Want to see more full solutions like this?
Chapter 12 Solutions
Principles of General, Organic, Biological Chemistry
- Identify the IUPAC name of the given structure. A. 2 - methylhexan-5-one B. 5 - methylhexan-2-one C. 2 - heptanone D. 5 - heptanone Identify the IUPAC name of the given structure. A. 4 - bromopentan-3-one B. 1 - bromobutan-2-one C. 2 - bromobutan-one D. None of the abovearrow_forward48arrow_forward1. What orbitals (around Carbon) are used to form each bond in the following molecules: A. CH3CH3 B. CH2CH2 2. Draw the structure corresponding to each IUPAC name: 3-ethyl-1,1-dimethylcyclohexane, 6-isopropyl-2,3-dimethylnonanearrow_forward
- give the IUPAC name for each compoundarrow_forwardThe correct IUPAC name for this compound is: O a. 4-oxa-1-cyclohexanol O b. 4-hydroxyoxacyclohexene O c. 4-oxa-1-cyclohexenol O d. 4-oxacyclohexane-1-ol OHarrow_forwardWhich alkyl halide has the highest boiling point? A. CH3BrB. CH3FC. CH3ClD. CH3larrow_forward
- 49arrow_forward1. Provide the IUPAC name for each of the following organic molecules. a. b. C. H3C H3C, Home H3C CH3 CH3 CH3 Br H 2. Draw structures for the following organic molecules, using the wedged/hashed line convention to depict any stereochemical relationships between groups: a. cis-3-ethylcyclobutane-1-carbaldehyde b. (R)-1-chloro-6-iodo-6-methyloctan-3-onearrow_forwardClassify each alkyl halide and alcohol as 1°, 2°, or 3°.arrow_forward
- 7 Give the IUPAC name for the following compound. OH A. 3-methyl-1-pentenyl alcohol B. 3-vinyl-1-butanol C. 3-methyl-4-penten-1-olarrow_forwardThe type of intermediate formed when HgSO4/H2SO4/H2O is added to an alkyne is: a.an alcohol b.an aldehyde c.an enol d.a diol e.a ketonearrow_forward30. What is the IUPAC name for this compound? CH3 0 1 I CH3-CH-CH₂-C-OH A. pentanoic acid B. 3-methylbutanoic acid C. 2-methylbutanoic acid D. 2-methyl-4-butanoic acidarrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





