
Concept explainers
(a)
Interpretation: To determine the number of NADH molecules formed during one turn of the citric acid cycle.
Concept introduction: Citric acid cycle is the third stage of the biochemical energy production process. The cycle includes the reactions in which the acetyl part of acetyl CoA is oxidized and leads to the formation of carbon dioxide and
(a)
Answer to Problem 12.70EP
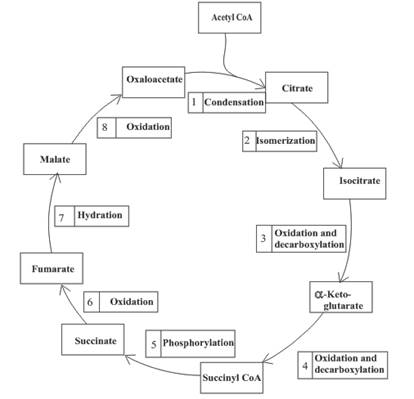
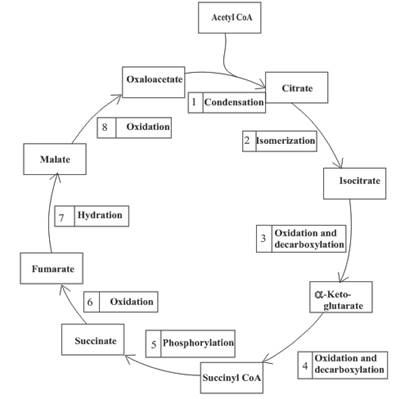
Three molecules of NADH are formed in step 3, 4 and 8 of the citric acid cycle.
Explanation of Solution
Step 3 is the first step where both the oxidation and decarboxylation occurs. Step 3 involves the oxidation of isocitrate and formation of CO2. In this step, firstly isocitrate is oxidized by
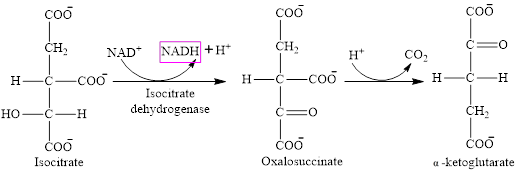
Step 4 involves the oxidation of

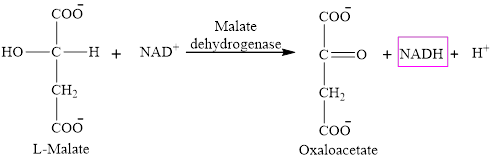
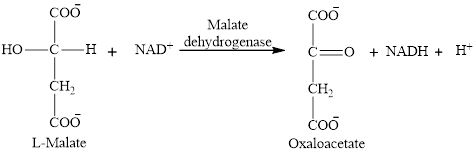
Step 8 is an oxidation reaction and the last step of the citric acid cycle. In step 8,
The reaction of step 8 is:
Hence, three molecules of NADH are formed in the citric acid cycle.
(b)
Interpretation: To determine the number of GTP molecules formed during one turn of the citric acid cycle.
Concept introduction: Citric acid cycle is the third stage of the biochemical energy production process. The cycle includes the reactions in which the acetyl part of acetyl CoA is oxidized and leads to the formation of carbon dioxide and
(b)
Answer to Problem 12.70EP
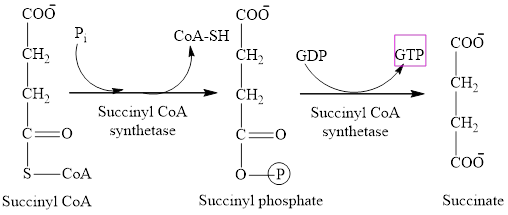
One molecule of GTP is formed in step 5 of the citric acid cycle.
Explanation of Solution
Step 5 involves the thioester bond cleavage in
(c)
Interpretation: To determine the number of time decarboxylation reactions occur during one turn of the citric acid cycle.
Concept introduction: Citric acid cycle is the third stage of the biochemical energy production process. The cycle includes the reactions in which the acetyl part of acetyl CoA is oxidized and leads to the formation of carbon dioxide and
(c)
Answer to Problem 12.70EP
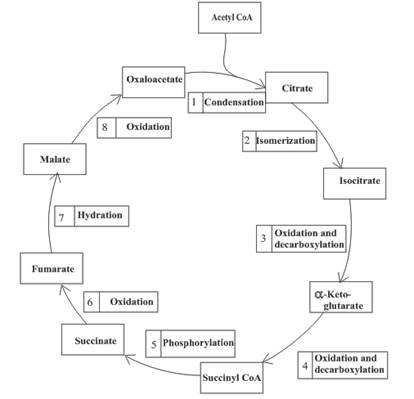
Decarboxylation occurs twice in the citric acid cycle in step 3 and 4.
Explanation of Solution
Step 3 is the first step where both the oxidation and decarboxylation occurs. Step 3 involves the oxidation of isocitrate and formation of CO2. In this step, firstly isocitrate is oxidized by
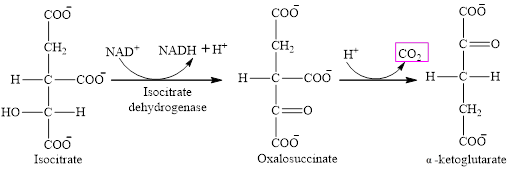
The final product is
Step 4 involves the oxidation of

The final product is
(d)
Interpretation: To determine the number of time oxidation-reduction reaction occur during one turn of the citric acid cycle.
Concept introduction: Citric acid cycle is the third stage of the biochemical energy production process. The cycle includes the reactions in which the acetyl part of acetyl CoA is oxidized and leads to the formation of carbon dioxide and
An overview of the citric acid cycle is as follows:
(d)
Answer to Problem 12.70EP
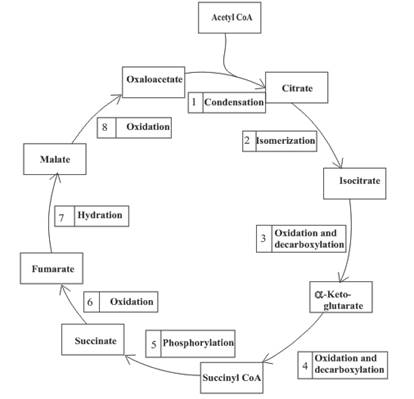
The oxidation-reduction reaction occurs four times in the citric acid cycle in step 3, 4, 6 and 8.
Explanation of Solution
Step 3 is the first step where both the oxidation and decarboxylation occurs. Step 3 involves the oxidation of isocitrate and formation of CO2. In this step, firstly isocitrate is oxidized by
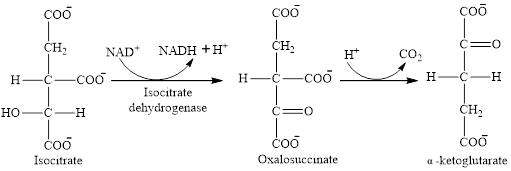
Step 4 involves the oxidation of

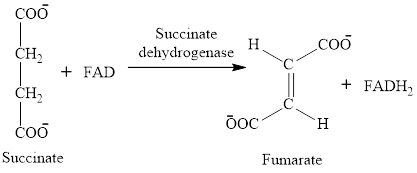
In step 6, oxidation of succinate occurs to form fumarate. The enzyme involved in this step of the citric acid cycle is succinate dehydrogenase. FAD is the oxidizing agent in this step. This reaction takes place in the inner mitochondrial membrane. The reaction of step 6 is:
Step 8 is an oxidation reaction and the last step of the citric acid cycle. In step 8,
The reaction of step 8 is:
Want to see more full solutions like this?
Chapter 12 Solutions
EBK ORGANIC AND BIOLOGICAL CHEMISTRY
- Draw the Fischer projection of D-fructose. Click and drag to start drawing a structure. Skip Part Check AP 14 tv SC F1 F2 80 F3 a F4 ! 2 # 3 CF F5 75 Ax MacBook Air 894 $ 5olo % Λ 6 > W F6 K F7 &arrow_forwardConsider this step in a radical reaction: Y What type of step is this? Check all that apply. Draw the products of the step on the right-hand side of the drawing area below. If more than one set of products is possible, draw any set. Also, draw the mechanism arrows on the left-hand side of the drawing area to show how this happens. ionization propagation initialization passivation none of the abovearrow_forward22.16 The following groups are ortho-para directors. (a) -C=CH₂ H (d) -Br (b) -NH2 (c) -OCHS Draw a contributing structure for the resonance-stabilized cation formed during elec- trophilic aromatic substitution that shows the role of each group in stabilizing the intermediate by further delocalizing its positive charge. 22.17 Predict the major product or products from treatment of each compound with Cl₁/FeCl₂- OH (b) NO2 CHO 22.18 How do you account for the fact that phenyl acetate is less reactive toward electro- philic aromatic substitution than anisole? Phenyl acetate Anisole CH (d)arrow_forward
- Show how to convert ethyl benzene to (a) 2,5-dichlorobenzoic acid and (b) 2,4-dichlorobenzoic acid.arrow_forwardHelp me solve this problem. Thank you in advance.arrow_forward22.7 Predict the monoalkylated products of the following reactions with benzene. (a) AlCl3 Ya (b) AlCl3 (c) H3PO4 (d) 22.8 Think-Pair-Share AICI3 The reaction below is a common electrophilic aromatic substitution. SO3 H₂SO4 SO₂H (a) Draw the reaction mechanism for this reaction using HSO,+ as the electrophile. (b) Sketch the reaction coordinate diagram, where the product is lower in energy than the starting reactant. (c) Which step in the reaction mechanism is highest in energy? Explain. (d) Which of the following reaction conditions could be used in an electrophilic aro- matic substitution with benzene to provide substituted phenyl derivatives? (i) AICI3 HNO3 H₂SO4 K2Cr2O7 (iii) H₂SO4 (iv) H₂PO₁arrow_forward
- Is an acid-base reaction the only type of reaction that would cause leavening products to rise?arrow_forwardHelp me understand this! Thank you in advance.arrow_forward22.22 For each compound, indicate which group on the ring is more strongly activating and then draw a structural formula of the major product formed by nitration of the compound. Br CHO (a) CH3 (b) (c) CHO CH3 SO₂H (d) ☑ OCHS NO₂ (e) (f) CO₂H NHCOCH3 NHCOCH, (h) CHS 22.23 The following molecules each contain two aromatic rings. (b) 000-100- H3C (a) (c) Which ring in each undergoes electrophilic aromatic substitution more readily? Draw the major product formed on nitration.arrow_forward
- V Consider this step in a radical reaction: Br: ? What type of step is this? Check all that apply. Draw the products of the step on the right-hand side of the drawing area below. If more than one set of products is possible, draw any set. Also, draw the mechanism arrows on the left-hand side of the drawing area to show how this happens. ⚫ionization termination initialization neutralization none of the abc Explanation Check 80 Ο F3 F1 F2 2 F4 01 % do5 $ 94 #3 X 5 C MacBook Air 25 F5 F6 66 ©2025 ˇ F7 29 & 7 8arrow_forwardShow how to convert ethyl benzene to (a) 2,5-dichlorobenzoic acid and (b) 2,4-dichlorobenzoic acid.arrow_forwardno aiarrow_forward
 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning, Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning Chemistry In FocusChemistryISBN:9781305084476Author:Tro, Nivaldo J., Neu, Don.Publisher:Cengage Learning
Chemistry In FocusChemistryISBN:9781305084476Author:Tro, Nivaldo J., Neu, Don.Publisher:Cengage Learning




