
EBK ORGANIC AND BIOLOGICAL CHEMISTRY
7th Edition
ISBN: 9780100547742
Author: STOKER
Publisher: YUZU
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Chapter 12, Problem 12.37EP
Interpretation Introduction
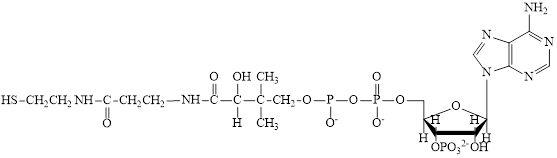
Interpretation: To draw the three-subunit block diagram for CoA.
Concept introduction: Coenzymes are non-protein organic compounds that are used along with the enzymes and help to carry forward the reaction. Coenzymes cannot perform on their own alone. CoA is also an example of the coenzyme.
Coenzyme A (CoA) is utilized in various
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Indicate the products of the reaction of 2-(3-aminopropyl)cyclohexan-1-one with H2SO4. Draw the structures of the compounds.
Indicate the products of the reaction of 2-cyclopentyl-2-methyl-1,3-dioxolane with H3O+. Draw the structures of the compounds.
Question 4 For the molecule shown below, (7 marks):
A) Sketch the Newman projection for the view looking along the bond from the
perspective of the arrow.
B) Then, draw the Newman projection for each 60° rotation along the bond until it
returns to the starting point.
C) Clearly indicate which Newman projection is the one we see in the structure shown
below, and clearly indicate which Newman projection is the highest in energy and
which is the lowest in energy.
H
H
Me
'H
Me
Me
Chapter 12 Solutions
EBK ORGANIC AND BIOLOGICAL CHEMISTRY
Ch. 12.1 - Prob. 1QQCh. 12.1 - Prob. 2QQCh. 12.2 - Which of the following is not found within the...Ch. 12.2 - Which of the following is not an organelle? a....Ch. 12.2 - Prob. 3QQCh. 12.2 - Which of the following statements about...Ch. 12.3 - Prob. 1QQCh. 12.3 - Prob. 2QQCh. 12.3 - Which of the following statements concerning...Ch. 12.3 - Which of the following statements concerning...
Ch. 12.3 - Which of the following statements concerning...Ch. 12.4 - Prob. 1QQCh. 12.4 - Prob. 2QQCh. 12.5 - Prob. 1QQCh. 12.5 - Prob. 2QQCh. 12.5 - Prob. 3QQCh. 12.6 - Which of the following occurs in the second stage...Ch. 12.6 - Which of the following stages in the biochemical...Ch. 12.6 - Prob. 3QQCh. 12.7 - Prob. 1QQCh. 12.7 - Prob. 2QQCh. 12.7 - Prob. 3QQCh. 12.7 - How many NADH and FADH2 molecules are produced,...Ch. 12.7 - Which of the following citric acid cycle...Ch. 12.7 - In which of the following listings of citric acid...Ch. 12.8 - Which of the following is a fuel for the electron...Ch. 12.8 - Which of the following is a mobile electron...Ch. 12.8 - What is the substrate that initially interacts...Ch. 12.8 - The number of fixed enzyme sites in the electron...Ch. 12.8 - Prob. 5QQCh. 12.8 - In which step in the electron transport chain does...Ch. 12.9 - Prob. 1QQCh. 12.9 - Prob. 2QQCh. 12.9 - Prob. 3QQCh. 12.10 - Prob. 1QQCh. 12.10 - Prob. 2QQCh. 12.11 - Prob. 1QQCh. 12.11 - Prob. 2QQCh. 12.11 - Prob. 3QQCh. 12.12 - How many different B vitamins participate in the...Ch. 12.12 - Prob. 2QQCh. 12 - Classify anabolism and catabolism as synthetic or...Ch. 12 - Classify anabolism and catabolism as...Ch. 12 - What is a metabolic pathway?Ch. 12 - Prob. 12.4EPCh. 12 - Classify each of the following processes as...Ch. 12 - Prob. 12.6EPCh. 12 - Prob. 12.7EPCh. 12 - Prob. 12.8EPCh. 12 - Prob. 12.9EPCh. 12 - Indicate whether each of the following statements...Ch. 12 - Prob. 12.11EPCh. 12 - Prob. 12.12EPCh. 12 - Prob. 12.13EPCh. 12 - Prob. 12.14EPCh. 12 - Prob. 12.15EPCh. 12 - Prob. 12.16EPCh. 12 - Prob. 12.17EPCh. 12 - Prob. 12.18EPCh. 12 - Prob. 12.19EPCh. 12 - Prob. 12.20EPCh. 12 - Prob. 12.21EPCh. 12 - Prob. 12.22EPCh. 12 - Prob. 12.23EPCh. 12 - Write a generalized chemical equation, containing...Ch. 12 - Prob. 12.25EPCh. 12 - Prob. 12.26EPCh. 12 - Prob. 12.27EPCh. 12 - Prob. 12.28EPCh. 12 - Prob. 12.29EPCh. 12 - Prob. 12.30EPCh. 12 - Prob. 12.31EPCh. 12 - Prob. 12.32EPCh. 12 - Prob. 12.33EPCh. 12 - Prob. 12.34EPCh. 12 - What identical structural subunits do the...Ch. 12 - Prob. 12.36EPCh. 12 - Prob. 12.37EPCh. 12 - Prob. 12.38EPCh. 12 - Prob. 12.39EPCh. 12 - Prob. 12.40EPCh. 12 - Prob. 12.41EPCh. 12 - Prob. 12.42EPCh. 12 - Classify each of the following molecules as (1) an...Ch. 12 - Prob. 12.44EPCh. 12 - Prob. 12.45EPCh. 12 - Prob. 12.46EPCh. 12 - Prob. 12.47EPCh. 12 - Prob. 12.48EPCh. 12 - Prob. 12.49EPCh. 12 - Prob. 12.50EPCh. 12 - Prob. 12.51EPCh. 12 - Prob. 12.52EPCh. 12 - Prob. 12.53EPCh. 12 - Prob. 12.54EPCh. 12 - Prob. 12.55EPCh. 12 - Prob. 12.56EPCh. 12 - Prob. 12.57EPCh. 12 - Prob. 12.58EPCh. 12 - List, by name, the four general stages of the...Ch. 12 - Which, by name, of the four general stages of the...Ch. 12 - Prob. 12.61EPCh. 12 - Prob. 12.62EPCh. 12 - Prob. 12.63EPCh. 12 - Prob. 12.64EPCh. 12 - Prob. 12.65EPCh. 12 - Prob. 12.66EPCh. 12 - Prob. 12.67EPCh. 12 - Prob. 12.68EPCh. 12 - Prob. 12.69EPCh. 12 - Prob. 12.70EPCh. 12 - Prob. 12.71EPCh. 12 - Prob. 12.72EPCh. 12 - Prob. 12.73EPCh. 12 - Prob. 12.74EPCh. 12 - Prob. 12.75EPCh. 12 - Prob. 12.76EPCh. 12 - Prob. 12.77EPCh. 12 - Prob. 12.78EPCh. 12 - Prob. 12.79EPCh. 12 - Prob. 12.80EPCh. 12 - Prob. 12.81EPCh. 12 - Prob. 12.82EPCh. 12 - Prob. 12.83EPCh. 12 - Prob. 12.84EPCh. 12 - Prob. 12.85EPCh. 12 - Prob. 12.86EPCh. 12 - Prob. 12.87EPCh. 12 - Prob. 12.88EPCh. 12 - Prob. 12.89EPCh. 12 - Indicate whether each of the following changes...Ch. 12 - Prob. 12.91EPCh. 12 - Prob. 12.92EPCh. 12 - Prob. 12.93EPCh. 12 - Prob. 12.94EPCh. 12 - Prob. 12.95EPCh. 12 - Prob. 12.96EPCh. 12 - Prob. 12.97EPCh. 12 - Prob. 12.98EPCh. 12 - Prob. 12.99EPCh. 12 - Prob. 12.100EPCh. 12 - Prob. 12.101EPCh. 12 - Prob. 12.102EPCh. 12 - Prob. 12.103EPCh. 12 - Prob. 12.104EPCh. 12 - Prob. 12.105EPCh. 12 - Prob. 12.106EPCh. 12 - Prob. 12.107EPCh. 12 - Prob. 12.108EPCh. 12 - Prob. 12.109EPCh. 12 - Prob. 12.110EPCh. 12 - Prob. 12.111EPCh. 12 - Prob. 12.112EPCh. 12 - Prob. 12.113EPCh. 12 - Prob. 12.114EPCh. 12 - Prob. 12.115EPCh. 12 - Prob. 12.116EPCh. 12 - Prob. 12.117EPCh. 12 - Prob. 12.118EPCh. 12 - Prob. 12.119EPCh. 12 - Prob. 12.120EPCh. 12 - Prob. 12.121EPCh. 12 - Prob. 12.122EPCh. 12 - Prob. 12.123EPCh. 12 - Prob. 12.124EPCh. 12 - Prob. 12.125EPCh. 12 - Prob. 12.126EPCh. 12 - Prob. 12.127EPCh. 12 - Prob. 12.128EPCh. 12 - Indicate whether or not each of the following B...Ch. 12 - Prob. 12.130EPCh. 12 - Prob. 12.131EPCh. 12 - Prob. 12.132EP
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Draw the major product of this reaction. Ignore inorganic byproducts and the amine side product. 'N' 1. NaOH, heat 2. Neutralizing work-up Select to Drawarrow_forwardSubmit Problem 3 of 10 Draw the major product of this reaction. Ignore inorganic byproducts and the amine side product. O 'N' NH 1. NaOH, heat 2. Neutralizing work-up Select to Drawarrow_forwardb) Certain cyclic compounds are known to be conformationally similar to carbohydrates, although they are not themselves carbohydrates. One example is Compound C shown below, which could be imagined as adopting four possible conformations. In reality, however, only one of these is particularly stable. Circle the conformation you expect to be the most stable, and provide an explanation to justify your choice. For your explanation to be both convincing and correct, it must contain not only words, but also "cartoon" orbital drawings contrasting the four structures. Compound C Possible conformations (circle one): Детarrow_forward
- Lab Data The distance entered is out of the expected range. Check your calculations and conversion factors. Verify your distance. Will the gas cloud be closer to the cotton ball with HCI or NH3? Did you report your data to the correct number of significant figures? - X Experimental Set-up HCI-NH3 NH3-HCI Longer Tube Time elapsed (min) 5 (exact) 5 (exact) Distance between cotton balls (cm) 24.30 24.40 Distance to cloud (cm) 9.70 14.16 Distance traveled by HCI (cm) 9.70 9.80 Distance traveled by NH3 (cm) 14.60 14.50 Diffusion rate of HCI (cm/hr) 116 118 Diffusion rate of NH3 (cm/hr) 175.2 175.2 How to measure distance and calculate ratearrow_forwardFor the titration of a divalent metal ion (M2+) with EDTA, the stoichiometry of the reaction is typically: 1:1 (one mole of EDTA per mole of metal ion) 2:1 (two moles of EDTA per mole of metal ion) 1:2 (one mole of EDTA per two moles of metal ion) None of the abovearrow_forwardPlease help me solve this reaction.arrow_forward
- Indicate the products obtained by mixing 2,2-dimethylpropanal with acetaldehyde and sodium ethoxide in ethanol.arrow_forwardSynthesize 2-Ethyl-3-methyloxirane from dimethyl(propyl)sulfonium iodide using the necessary organic or inorganic reagents. Draw the structures of the compounds.arrow_forwardSynthesize 2-Hydroxy-2-phenylacetonitrile from phenylmethanol using the necessary organic or inorganic reagents. Draw the structures of the compounds.arrow_forward
- Synthesize N-Methylcyclohexylamine from cyclohexanol using the necessary organic or inorganic reagents. Draw the structures of the compounds.arrow_forwardSynthesize N-Methylcyclohexylamine from cyclohexanol using the necessary organic or inorganic reagents. Draw the structures of the compounds.arrow_forwardIf possible, please provide the formula of the compound 3,3-dimethylbut-2-enal.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning, Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
 Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning

General, Organic, and Biological Chemistry
Chemistry
ISBN:9781285853918
Author:H. Stephen Stoker
Publisher:Cengage Learning

Organic And Biological Chemistry
Chemistry
ISBN:9781305081079
Author:STOKER, H. Stephen (howard Stephen)
Publisher:Cengage Learning,

Chemistry for Today: General, Organic, and Bioche...
Chemistry
ISBN:9781305960060
Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:Cengage Learning


Introduction to General, Organic and Biochemistry
Chemistry
ISBN:9781285869759
Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:Cengage Learning