
Concept explainers
(a)
Interpretation: To identify the substances ATP, CoA–SH,
Concept introduction: The sum of various
ATP is a
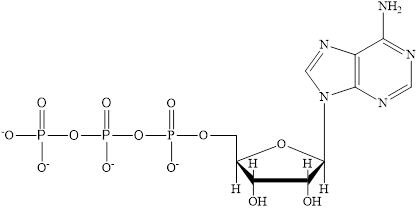
Coenzyme A (CoA) is a coenzyme which is utilized in various metabolic reactions. The functions of coenzyme A include oxidation of pyruvate in the citric cycle and fatty acid oxidation. The structure of Coenzyme A (CoA) is:
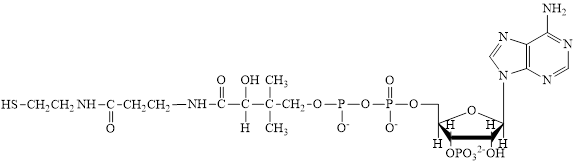
Flavin adenine dinucleotide exists in two forms: oxidized form
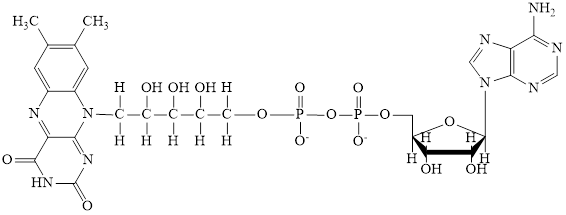
Nicotinamide adenine dinucleotide
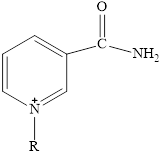
Here,
(a)
Answer to Problem 12.45EP
Explanation of Solution
The structure of
The structure of

Here,
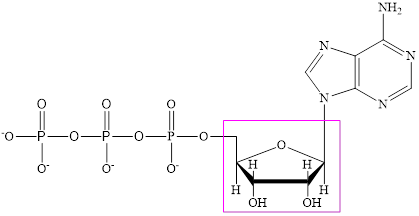
The structure of ATP is:
The structure of coenzyme A (CoA) is:
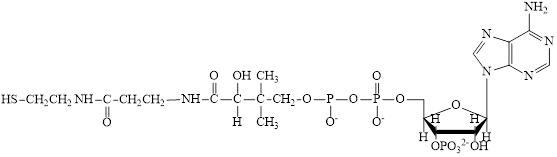
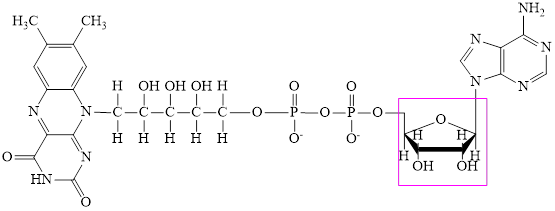
The structure of
The ribose subunit in each of the metabolic intermediate is highlighted. Here, the structure of
(b)
Interpretation: To identify the substances ATP, CoA–SH,
Concept introduction: The sum of various chemical reactions occurring in the human body is called metabolism and the reactions individually are known as metabolic reactions. During these metabolic reactions, the various metabolic intermediates are formed for the short time to complete the reactions. ATP,
ATP is a nucleotide which provides energy for the completion of various metabolic reactions occurring in our human body. The structure of ATP consists of adenine base, ribose sugar unit and the three phosphate group connected to each other by phosphoanhydride bonds. The structure of ATP is:

Coenzyme A (CoA) is a coenzyme which is utilized in various metabolic reactions. The functions of coenzyme A include oxidation of pyruvate in the citric cycle and fatty acid oxidation. The structure of Coenzyme A (CoA) is:

Flavin adenine dinucleotide exists in two forms: oxidized form

Nicotinamide adenine dinucleotide

Here,
(b)
Answer to Problem 12.45EP
CoA-SH,
Explanation of Solution
The structure of CoA-SH is:
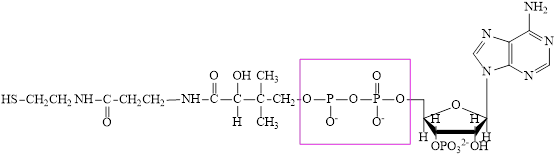
The structure of
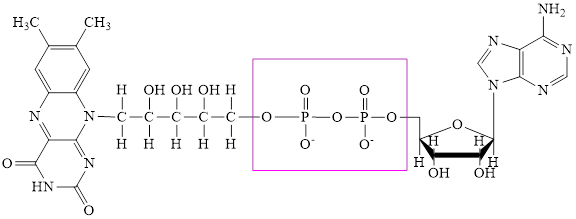
The structure of
Here,
The structure of
The structure of ATP is:
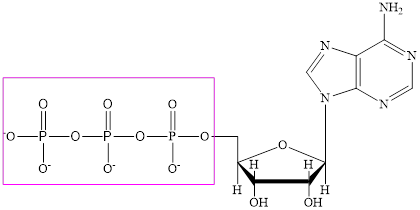
The phosphate subunit in each of the metabolic intermediate is highlighted. Here, the structure of
(c)
Interpretation: To identify the substances ATP, CoA–SH,
Concept introduction: The sum of various chemical reactions occurring in the human body is called metabolism and the reactions individually are known as metabolic reactions. During these metabolic reactions, the various metabolic intermediates are formed for the short time to complete the reactions. ATP,
ATP is a nucleotide which provides energy for the completion of various metabolic reactions occurring in our human body. The structure of ATP consists of adenine base, ribose sugar unit and the three phosphate group connected to each other by phosphoanhydride bonds. The structure of ATP is:

Coenzyme A (CoA) is a coenzyme which is utilized in various metabolic reactions. The functions of coenzyme A include oxidation of pyruvate in the citric cycle and fatty acid oxidation. The structure of Coenzyme A (CoA) is:

Flavin adenine dinucleotide exists in two forms: oxidized form

Nicotinamide adenine dinucleotide
The structure of

Here,
(c)
Answer to Problem 12.45EP
CoA-SH,
Explanation of Solution
The structure of CoA-SH is:
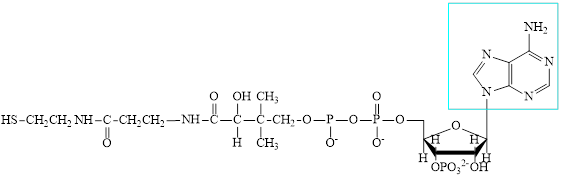
The structure of
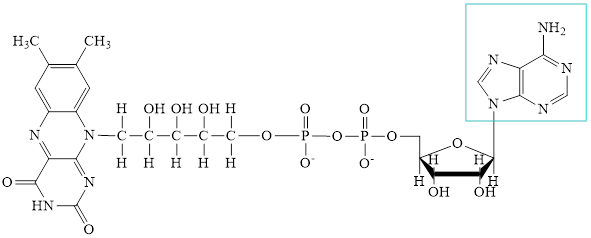
The structure of

Here,
ADP is a nucleotide which further consists of adenine base, ribose sugar unit and the two phosphate group.
The structure of ATP is:
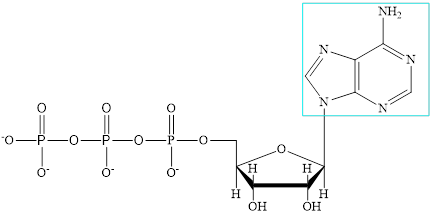
The adenine subunit in each of the metabolic intermediate is highlighted. Here, the structure of
(d)
Interpretation: To identify the substances ATP, CoA–SH,
Concept introduction: The sum of various chemical reactions occurring in the human body is called metabolism and the reactions individually are known as metabolic reactions. During these metabolic reactions, the various metabolic intermediates are formed for the short time to complete the reactions. ATP,
ATP is a nucleotide which provides energy for the completion of various metabolic reactions occurring in our human body. The structure of ATP consists of adenine base, ribose sugar unit and the three phosphate group connected to each other by phosphoanhydride bonds. The structure of ATP is:

Coenzyme A (CoA) is a coenzyme which is utilized in various metabolic reactions. The functions of coenzyme A include oxidation of pyruvate in the citric cycle and fatty acid oxidation. The structure of Coenzyme A (CoA) is:
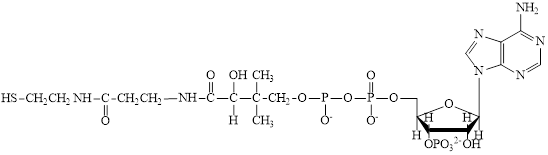
Flavin adenine dinucleotide exists in two forms: oxidized form

Nicotinamide adenine dinucleotide

Here,
(d)
Answer to Problem 12.45EP
CoA-SH,
Explanation of Solution
The structure of CoA-SH is:
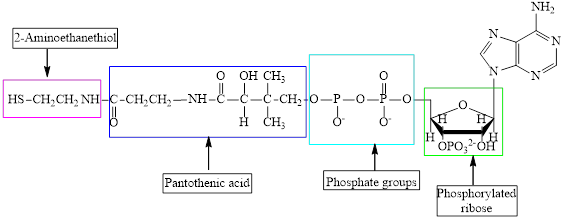
The structure of
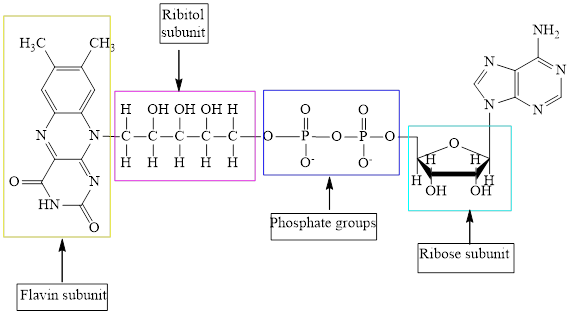
The structure of

Here,
The structure of ATP is:
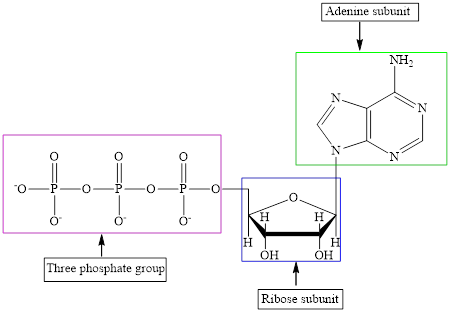
The different kinds of the subunits in metabolic intermediate are highlighted. Here, the structure of
Want to see more full solutions like this?
Chapter 12 Solutions
EBK ORGANIC AND BIOLOGICAL CHEMISTRY
- Select the major product of the following reaction. Br Br₂, light D Br Br Br Brarrow_forwardSelect all molecules which are chiral. Brarrow_forwardUse the reaction coordinate diagram to answer the below questions. Type your answers into the answer box for each question. (Watch your spelling) Energy A B C D Reaction coordinate E A) Is the reaction step going from D to F endothermic or exothermic? A F G B) Does point D represent a reactant, product, intermediate or transition state? A/ C) Which step (step 1 or step 2) is the rate determining step? Aarrow_forward
- 1. Using radii from Resource section 1 (p.901) and Born-Lande equation, calculate the lattice energy for PbS, which crystallizes in the NaCl structure. Then, use the Born-Haber cycle to obtain the value of lattice energy for PbS. You will need the following data following data: AH Pb(g) = 196 kJ/mol; AHƒ PbS = −98 kJ/mol; electron affinities for S(g)→S¯(g) is -201 kJ/mol; S¯(g) (g) is 640kJ/mol. Ionization energies for Pb are listed in Resource section 2, p.903. Remember that enthalpies of formation are calculated beginning with the elements in their standard states (S8 for sulfur). The formation of S2, AHF: S2 (g) = 535 kJ/mol. Compare the two values, and explain the difference. (8 points)arrow_forwardIn the answer box, type the number of maximum stereoisomers possible for the following compound. A H H COH OH = H C Br H.C OH CHarrow_forward7. Magnesium is found in nature in the form of carbonates and sulfates. One of the major natural sources of zinc is zinc blende (ZnS). Use relevant concepts of acid-base theory to explain this combination of cations and anions in these minerals. (2 points)arrow_forward
- 6. AlF3 is insoluble in liquid HF but dissolves if NaF is present. When BF3 is added to the solution, AlF3 precipitates. Write out chemical processes and explain them using the principles of Lewis acid-base theory. (6 points)arrow_forward5. Zinc oxide is amphoteric. Write out chemical reactions for dissolution of ZnO in HCl(aq) and in NaOH(aq). (3 points)arrow_forwardDraw the product(s) formed when alkene A is reacted with ozone, followed by Zn and H₂O. If no second product is formed, do not draw a structure in the second box. Higher Molecular Weight Product A Lower Molecular Weight Product draw structure ... draw structure ...arrow_forward
- Rank A - D in order of increasing rate of reaction with H2 and Pd/C. ب ب ب ب A B с Which option correctly ranks the alkenes in order of increasing rate of reaction with H₂ and Pd/C? О Barrow_forwardDraw the product of the following Sharpless epoxidation, including stereochemistry. Click the "draw structure" button to launch the drawing utility. -OH (CH3)3C-OOH Ti[OCH(CH3)2]4 (+)-DET draw structure ... Guidarrow_forwardWhat alkyne (or diyne) yields the following oxidative cleavage products? Click the "draw structure" button to launch the drawing utility. draw structure ... CO₂ + OHarrow_forwardarrow_back_iosSEE MORE QUESTIONSarrow_forward_ios
 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning, Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning Chemistry In FocusChemistryISBN:9781305084476Author:Tro, Nivaldo J., Neu, Don.Publisher:Cengage Learning
Chemistry In FocusChemistryISBN:9781305084476Author:Tro, Nivaldo J., Neu, Don.Publisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning





