
Organic Chemistry
8th Edition
ISBN: 9781305580350
Author: William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
Chapter 11.9, Problem DQ
Interpretation Introduction
Interpretation:
The relative amount of trans-tetrol to cis-tetrol at
Concept Introduction:
Ring opening: Ring opening of epoxide takes place by acid-catalyzed hydrolysis of an epoxide.
Acid-catalyzed hydrolysis of an epoxide via
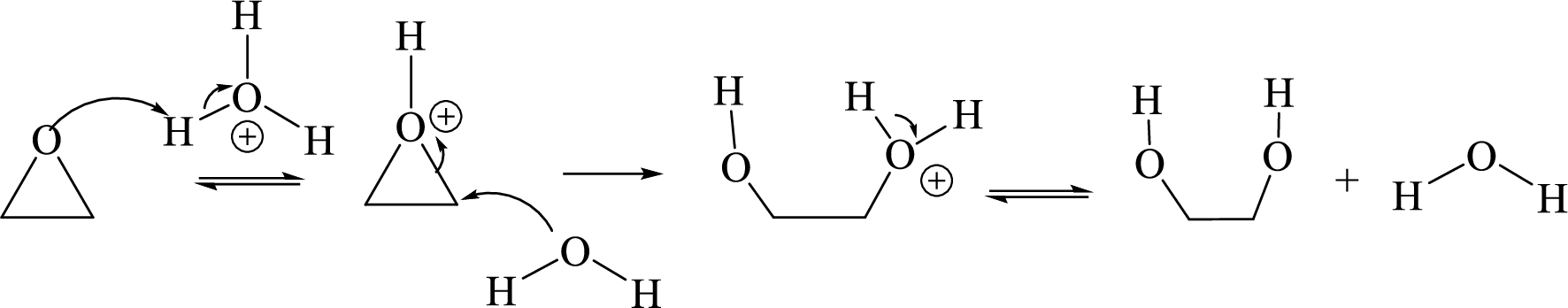
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
12
Mass Spectrometry
(d) This unknown contains oxygen, but it does not show any significant infrared absorption
peaks above 3000 cm .
59
100-
BO
40
Relative Abundance
M(102)
-
15 20
25 30 35 40 45 50 5
60 65 70 75 80
85 90 95 100 105
miz
Draw a Haworth projection of a common cyclic form of this monosaccharide:
H
HO
H
HO
H
HO
H
H
-OH
CH2OH
Click and drag to start drawing a
structure.
Х
:
D
:
Draw the structure of valylasparagine, a dipeptide made from valine and asparagine, as it would appear at physiological pH.
Click and drag to start drawing a
structure.
P
D
Chapter 11 Solutions
Organic Chemistry
Ch. 11.2 - Write IUPAC and common names for these ethers. (a)...Ch. 11.3 - Arrange these compounds in order of increasing...Ch. 11.4 - Show how you might use the Williamson ether...Ch. 11.4 - Show how ethyl hexyl ether might be prepared by a...Ch. 11.5 - Account for the fact that treatment of tert-butyl...Ch. 11.5 - Draw structural formulas for the major products of...Ch. 11.6 - Prob. 11.7PCh. 11.8 - Draw the expected products of Sharpless...Ch. 11.9 - Prob. AQCh. 11.9 - Prob. BQ
Ch. 11.9 - Prob. CQCh. 11.9 - Prob. DQCh. 11 - Write names for these compounds. Where possible,...Ch. 11 - Prob. 11.11PCh. 11 - Each compound given in this problem is a common...Ch. 11 - Account for the fact that tetrahydrofuran (THF) is...Ch. 11 - Prob. 11.14PCh. 11 - Write equations to show a combination of reactants...Ch. 11 - Propose a mechanism for this reaction.Ch. 11 - Prob. 11.17PCh. 11 - Prob. 11.18PCh. 11 - Prob. 11.19PCh. 11 - Prob. 11.20PCh. 11 - Ethylene oxide is the starting material for the...Ch. 11 - Prob. 11.22PCh. 11 - Predict the structural formula of the major...Ch. 11 - The following equation shows the reaction of...Ch. 11 - Propose a mechanism to account for this...Ch. 11 - Acid-catalyzed hydrolysis of the following epoxide...Ch. 11 - Prob. 11.27PCh. 11 - Prob. 11.28PCh. 11 - Prob. 11.29PCh. 11 - Propose a mechanism for the following...Ch. 11 - Show reagents and experimental conditions to...Ch. 11 - Starting with cis-3-hexene, show how to prepare...Ch. 11 - Show reagents to convert cycloheptene to each of...Ch. 11 - Show reagents to convert bromocyclopentane to each...Ch. 11 - Prob. 11.35PCh. 11 - Starting with acetylene and ethylene oxide as the...Ch. 11 - Following are the steps in the industrial...Ch. 11 - Prob. 11.38PCh. 11 - Prob. 11.39PCh. 11 - Aldehydes and ketones react with one molecule of...Ch. 11 - Prob. 11.42PCh. 11 - Write the products of the following sequences of...Ch. 11 - Using your reaction roadmap as a guide, show how...Ch. 11 - Using your reaction roadmap as a guide, show how...Ch. 11 - Using your reaction roadmap as a guide, show how...Ch. 11 - During the synthesis of the antiasthmatic drug...Ch. 11 - Prob. 11.48P
Knowledge Booster
Similar questions
- Draw the Haworth projection of α-L-mannose. You will find helpful information in the ALEKS Data resource. Click and drag to start drawing a structure. : ཊི Х Darrow_forwardDraw the structure of serine at pH 6.8. Click and drag to start drawing a structure. : d كarrow_forwardTake a look at this molecule, and then answer the questions in the table below it. CH2OH H H H OH OH OH CH2OH H H H H OH H H OH H OH Is this a reducing sugar? yes α β ロ→ロ no ☑ yes Does this molecule contain a glycosidic bond? If you said this molecule does contain a glycosidic bond, write the symbol describing it. O no 0+0 If you said this molecule does contain a glycosidic bond, write the common names (including anomer and enantiomer labels) of the molecules that would be released if that bond were hydrolyzed. If there's more than one molecule, separate each name with a comma. ☐arrow_forward
- Answer the questions in the table below about this molecule: H₂N-CH₂ -C—NH–CH–C—NH–CH—COO- CH3 CH CH3 What kind of molecule is this? 0= CH2 C If you said the molecule is a peptide, write a description of it using 3-letter codes separated ☐ by dashes. polysaccharide peptide amino acid phospolipid none of the above Хarrow_forwardDraw a Haworth projection of a common cyclic form of this monosaccharide: CH₂OH C=O HO H H -OH H OH CH₂OH Click and drag to start drawing a structure. : ☐ Х S '☐arrow_forwardNucleophilic Aromatic Substitution 22.30 Predict all possible products formed from the following nucleophilic substitution reactions. (a) (b) 9 1. NaOH 2. HCI, H₂O CI NH₁(!) +NaNH, -33°C 1. NaOH 2. HCl, H₂Oarrow_forward
- Syntheses 22.35 Show how to convert toluene to these compounds. (a) -CH,Br (b) Br- -CH3 22.36 Show how to prepare each compound from 1-phenyl-1-propanone. 1-Phenyl-1-propanone ہتی. Br. (b) Br (racemic) 22.37 Show how to convert ethyl benzene to (a) 2,5-dichlorobenzoic acid and (b) 2,4-dichlorobenzoic acid. 22.38 Show reagents and conditions to bring about the following conversions. (a) 9 NH2 8 CO₂H NH2 CO₂Et (d) NO2 NH2 S NH₂ NO2 CHS CHarrow_forwardive the major organic product(s) of each of the following reactions or sequences of reactions. Show all rant stereochemistry. [10 only] A. B. NaN3 1. LiAlH4, ether Br 2. H₂O CH3 HNO3 H₂/Pt H₂SO ethanol C. 0 0 CH3CC1 NaOH NHCCH AICI H₂O . NH₂ CH3CH2 N CH2CH3 + HCI CH₂CH 3 1. LIAIH, THE 2. H₂Oarrow_forwardCalculate the stoichiometric amount of CaCl2 needed to convert all of the CuSO4 into CuCl2.arrow_forward
- H CH تنی Cl 1. NaCN, DMF 2. LIAIH4, ether H₂O pyridine N NH₂ 5 CH H 1 HNO, H₂SO 2. Nal NH2 Br Br HNO₂ CuCl H₂SO HCI CH3 H3C NN HSO KCN CuCN 1. HNO₂, H₂SO O₂N NH2 2. OH ཀ་ལས། །ས་ཅན་ :i་དེ་མ་མ་སེ་ NH₂ CH3 1. HNO₂, H₂SO4 2. H3PO₂ 1 HNO2, H2SO4 2. Nalarrow_forwardive the major organic product(s) of each of the following reactions or sequences of reactions. Show all rant stereochemistry. [10 only] A. B. NaN3 1. LiAlH4, ether Br 2. H₂O CH3 HNO3 H₂/Pt H₂SO ethanol C. 0 0 CH3CC1 NaOH NHCCH AICI H₂O . NH₂ CH3CH2 N CH2CH3 + HCI CH₂CH 3 1. LIAIH, THE 2. H₂Oarrow_forwardIf a pharmacy chain sold 65 million 500-mg tablets of aspirin, how many US tons of aspirin does this represent? Report your answer to 2 significant figures.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY