
Concept explainers
(a)
Interpretation:
Structural formula for the given name has to be drawn.
Concept Introduction:
Nomenclature of Ether compounds:
The naming of theorganic compound is given by IUPAC (International Union for pure and applied chemistry). In the IUPAC names consist of certain rules for giving chemical names they are,
Ethers are named by selecting the longest carbon chain as the parent
Common name: List the alkyl groups bonded to Oxygen in alphabetical order and adding the word ‘ether’.
Cyclic ether: The presence of Oxygen atom in a saturated ring is indicated by the prefix ox-, and ring sizes from three to six are indicated by the endings –irane, etane, olane, and –ane, respectively.
Numbering of the atoms of the ring begins with the oxygen atom. These compounds and others in which there is a heteroatom (non-carbon atom) in the ring are called heterocycles.
(a)
Explanation of Solution
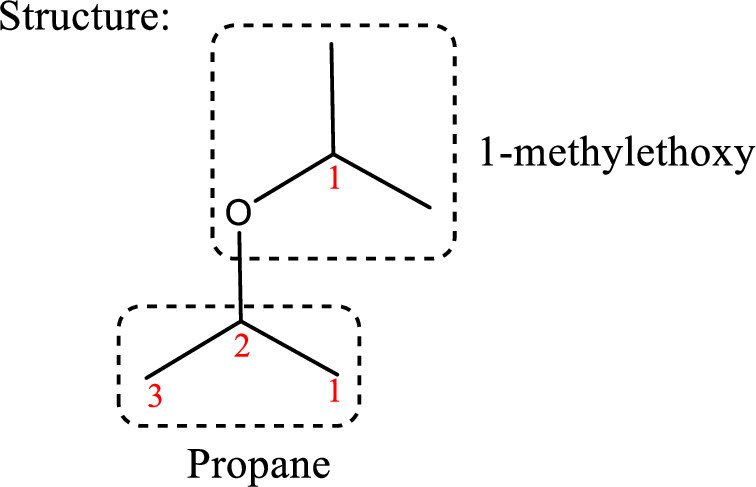
IUPAC name: 2-(1-Methylethoxy)propane
Ethers are named by selecting the longest carbon chain as the parent alkane and naming the –OR group bonded to it an alkoxy group.
Using the above principle, the longest carbon chain is propane (contains three carbons) as the parent alkane; naming the –OR group as alkoxy that is ‘2-(1-methylethoxy).
Therefore, the structure obtained is,
(b)
Interpretation:
Structural formula for the given name has to be drawn.
Concept Introduction:
Nomenclature of Ether compounds:
The naming of theorganic compound is given by IUPAC (International Union for pure and applied chemistry). In the IUPAC names consist of certain rules for giving chemical names they are,
Ethers are named by selecting the longest carbon chain as the parent alkane and naming the –OR group bonded to it an alkoxy group.
Common name: List the alkyl groups bonded to Oxygen in alphabetical order and adding the word ‘ether’.
Cyclic ether: The presence of Oxygen atom in a saturated ring is indicated by the prefix ox-, and ring sizes from three to six are indicated by the endings –irane, etane, olane, and –ane, respectively.
Numbering of the atoms of the ring begins with the oxygen atom. These compounds and others in which there is a heteroatom (non-carbon atom) in the ring are called heterocycles.
(b)
Explanation of Solution
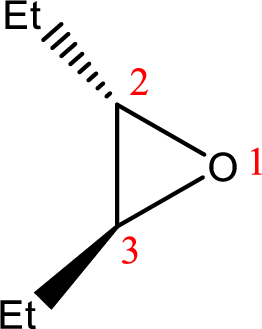
IUPAC name: trans-2,3-Diethyloxirane
Cyclic ether: The presence of Oxygen atom in a saturated ring is indicated by the prefix ox-, and ring sizes from three to six are indicated by the endings –irane, etane, olane, and –ane, respectively.
Numbering of the atoms of the ring begins with the oxygen atom. These compounds and others in which there is a heteroatom (non-carbon atom) in the ring are called heterocycles.
Using the above principle, the name‘oxirane’ contains two carbons along with one Oxygen atom in a cyclic ringalong with two ‘ethyl’ substituentsin the ring.
(c)
Interpretation:
Structural formula for the given name has to be drawn.
Concept Introduction:
Nomenclature of Ether compounds:
The naming of theorganic compound is given by IUPAC (International Union for pure and applied chemistry). In the IUPAC names consist of certain rules for giving chemical names they are,
Ethers are named by selecting the longest carbon chain as the parent alkane and naming the –OR group bonded to it an alkoxy group.
Common name: List the alkyl groups bonded to Oxygen in alphabetical order and adding the word ‘ether’.
Cyclic ether: The presence of Oxygen atom in a saturated ring is indicated by the prefix ox-, and ring sizes from three to six are indicated by the endings –irane, etane, olane, and –ane, respectively.
Numbering of the atoms of the ring begins with the oxygen atom. These compounds and others in which there is a heteroatom (non-carbon atom) in the ring are called heterocycles.
(c)
Explanation of Solution
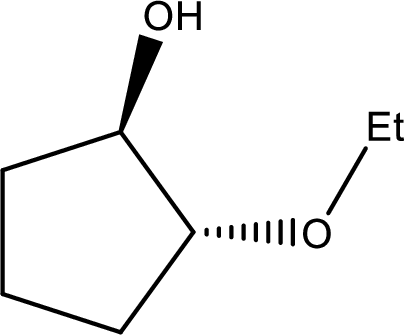
IUPAC name:trans-2-Ethoxycyclopentanol
Ethers are named by selecting the longest carbon chain as the parent alkane and naming the –OR group bonded to it an alkoxy group.
Here, the –OH group is preferred as the first priority and the compound is ‘alcohol’.
In the given compound, the longest carbon chain contains five carbons in a cyclic ring; the parent name is CYCLOPETANE and numbering the parent chain begins with carbon attached to the –OH group located at C-1 and the substituent ‘ethoxy’ is located at C-2.
Therefore, the structure is,
(d)
Interpretation:
Structural formula for the given name has to be drawn.
Concept Introduction:
Nomenclature of Ether compounds:
The naming of theorganic compound is given by IUPAC (International Union for pure and applied chemistry). In the IUPAC names consist of certain rules for giving chemical names they are,
Ethers are named by selecting the longest carbon chain as the parent alkane and naming the –OR group bonded to it an alkoxy group.
Common name: List the alkyl groups bonded to Oxygen in alphabetical order and adding the word ‘ether’.
Cyclic ether: The presence of Oxygen atom in a saturated ring is indicated by the prefix ox-, and ring sizes from three to six are indicated by the endings –irane, etane, olane, and –ane, respectively.
Numbering of the atoms of the ring begins with the oxygen atom. These compounds and others in which there is a heteroatom (non-carbon atom) in the ring are called heterocycles.
(d)
Explanation of Solution
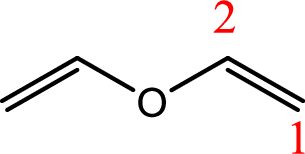
IUPAC name:Ethenyloxyethene.
Ethers are named by selecting the longest carbon chain as the parent alkane and naming the –OR group bonded to it an alkoxy group.
Using the above principle, the longest carbon chain isethene (contains two carbons with a double bond) as the parent
(e)
Interpretation:
Structural formula for the given name has to be drawn.
Concept Introduction:
Nomenclature of Ether compounds:
The naming of theorganic compound is given by IUPAC (International Union for pure and applied chemistry). In the IUPAC names consist of certain rules for giving chemical names they are,
Ethers are named by selecting the longest carbon chain as the parent alkane and naming the –OR group bonded to it an alkoxy group.
Common name: List the alkyl groups bonded to Oxygen in alphabetical order and adding the word ‘ether’.
Cyclic ether: The presence of Oxygen atom in a saturated ring is indicated by the prefix ox-, and ring sizes from three to six are indicated by the endings –irane, etane, olane, and –ane, respectively.
Numbering of the atoms of the ring begins with the oxygen atom. These compounds and others in which there is a heteroatom (non-carbon atom) in the ring are called heterocycles.
(e)
Explanation of Solution
IUPAC name:Cyclohexene oxide:
Cyclic ether: The presence of Oxygen atom in a saturated ring is indicated by the prefix ox-, and ring sizes from three to six are indicated by the endings –irane, etane, olane, and –ane, respectively.
Numbering of the atoms of the ring begins with the oxygen atom. These compounds and others in which there is a heteroatom (non-carbon atom) in the ring are called heterocycles.
Using the above principle, the longest carbon cyclic ring contains sixcarbon atoms andone Oxygen atom is located at C-1 and 2 as three membered ring.
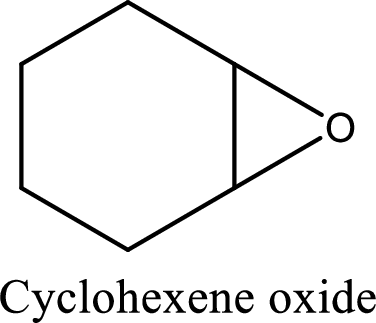
(f)
Interpretation:
Structural formula for the given name has to be drawn.
Concept Introduction:
Nomenclature of Ether compounds:
The naming of theorganic compound is given by IUPAC (International Union for pure and applied chemistry). In the IUPAC names consist of certain rules for giving chemical names they are,
Ethers are named by selecting the longest carbon chain as the parent alkane and naming the –OR group bonded to it an alkoxy group.
Common name: List the alkyl groups bonded to Oxygen in alphabetical order and adding the word ‘ether’.
Cyclic ether: The presence of Oxygen atom in a saturated ring is indicated by the prefix ox-, and ring sizes from three to six are indicated by the endings –irane, etane, olane, and –ane, respectively.
Numbering of the atoms of the ring begins with the oxygen atom. These compounds and others in which there is a heteroatom (non-carbon atom) in the ring are called heterocycles.
(f)
Explanation of Solution
IUPAC name:3-Cyclopropyloxy-1-propene
Ethers are named by selecting the longest carbon chain as the parent alkane and naming the –OR group bonded to it an alkoxy group.
Using the above principle, the longest carbon chain ispropene (contains three carbons with a double bond) as the parent alkene; naming the–OR groups as alkoxy that is 3-Cyclopropyloxy.
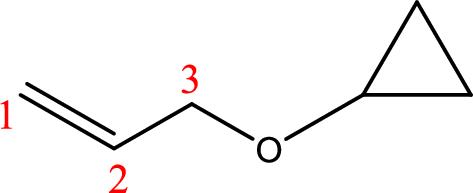
(g)
Interpretation:
Structural formula for the given name has to be drawn.
Concept Introduction:
Nomenclature of Ether compounds:
The naming of theorganic compound is given by IUPAC (International Union for pure and applied chemistry). In the IUPAC names consist of certain rules for giving chemical names they are,
Ethers are named by selecting the longest carbon chain as the parent alkane and naming the –OR group bonded to it an alkoxy group.
Common name: List the alkyl groups bonded to Oxygen in alphabetical order and adding the word ‘ether’.
Cyclic ether: The presence of Oxygen atom in a saturated ring is indicated by the prefix ox-, and ring sizes from three to six are indicated by the endings –irane, etane, olane, and –ane, respectively.
Numbering of the atoms of the ring begins with the oxygen atom. These compounds and others in which there is a heteroatom (non-carbon atom) in the ring are called heterocycles.
(g)
Explanation of Solution
IUPAC name:(R)-2-Methyloxirane
Cyclic ether: The presence of Oxygen atom in a saturated ring is indicated by the prefix ox-, and ring sizes from three to six are indicated by the endings –irane, etane, olane, and –ane, respectively.
Numbering of the atoms of the ring begins with the oxygen atom. These compounds and others in which there is a heteroatom (non-carbon atom) in the ring are called heterocycles.
Using the above principle, the longest three membered cyclic ringscontaintwo carbon atoms and one Oxygen atom and the substituent methyl group is located at C-2.
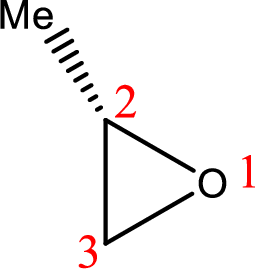
(h)
Interpretation:
Structural formula for the given name has to be drawn.
Concept Introduction:
Nomenclature of Ether compounds:
The naming of theorganic compound is given by IUPAC (International Union for pure and applied chemistry). In the IUPAC names consist of certain rules for giving chemical names they are,
Ethers are named by selecting the longest carbon chain as the parent alkane and naming the –OR group bonded to it an alkoxy group.
Common name: List the alkyl groups bonded to Oxygen in alphabetical order and adding the word ‘ether’.
Cyclic ether: The presence of Oxygen atom in a saturated ring is indicated by the prefix ox-, and ring sizes from three to six are indicated by the endings –irane, etane, olane, and –ane, respectively.
Numbering of the atoms of the ring begins with the oxygen atom. These compounds and others in which there is a heteroatom (non-carbon atom) in the ring are called heterocycles.
(h)
Explanation of Solution
IUPAC name:1,1-Dimethyloxycyclohexane,
Ethers are named by selecting the longest carbon chain as the parent alkane and naming the –OR group bonded to it an alkoxy group.
Using the above principle, the longest carbon ringiscyclohexane (contains six carbons) as the parent alkane; naming the two–OR groups as alkoxy that bothmethoxy groups located at C-1.
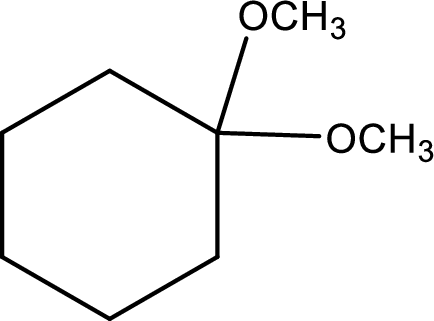
Want to see more full solutions like this?
Chapter 11 Solutions
Organic Chemistry
- Below is the SN1 reaction of (S)-3-chlorocyclohexene and hydroxide (OH). Draw the missing curved arrows, lone pairs of electrons, and nonzero formal charges. In the third box, draw the two enantiomeric products that will be produced. 5th attempt Please draw all four bonds at chiral centers. Draw the two enantiomeric products that will be produced. Draw in any hydrogen at chiral centers. 1000 4th attempt Feedback Please draw all four bonds at chiral centers. 8. R5 HO: See Periodic Table See Hint H Cl Br Jid See Periodic Table See Hintarrow_forwardShow that a molecule with configuration π4 has a cylindrically symmetric electron distribution. Hint: Let the π orbitals be equal to xf and yf, where f is a function that depends only on the distance from the internuclear axis.arrow_forward(a) Verify that the lattice energies of the alkali metal iodides are inversely proportional to the distances between the ions in MI (M = alkali metal) by plotting the lattice energies given below against the internuclear distances dMI. Is the correlation good? Would a better fit be obtained by plotting the lattice energies as a function of (1 — d*/d)/d, as theoretically suggested, with d* = 34.5 pm? You must use a standard graphing program to plot the graph. It generates an equation for the line and calculates a correlation coefficient. (b) From the graph obtained in (a), estimate the lattice energy of silver iodide. (c) Compare the results of (b) with the experimental value of 886 kJ/mol. If they do not agree, explain the deviation.arrow_forward
- Can I please get help with #3 & 4? Thanks you so much!arrow_forwardA solution consisting of 0.200 mol methylbenzene, C,H,CH,, in 500. g of nitrobenzene, CH,NO₂, freezes at 3.2°C. Pure nitrobenzene freezes at 6.0°C. The molal freezing point constant of nitrobenzene is _ °C/m. a) 2.8 b) 3.2 c) 5.6 d) 7.0 e) 14.0arrow_forwardBelow is the SN1 reaction of (S)-3-chlorocyclohexene and hydroxide ("OH). Draw the missing curved arrows, lone pairs of electrons, and nonzero formal charges. In the third box, draw the two enantiomeric products that will be produced. 2nd attempt Please draw all four bonds at chiral centers. 0 D Draw the missing curved arrow notation. Add lone pairs of electrons and nonzero formal charges. + 노 V 1st attempt Feedback Please draw all four bonds at chiral centers. See Periodic Table See Hint F P 41 H Br See Periodic Table See Hint H Larrow_forward
- How close are the Mulliken and Pauling electronegativity scales? (a) Now that the ionization energies and electron affinities have been defined, calculate the Mulliken and Pauling electronegativities for C, N, O and F. Compare them. (Make the necessary adjustments to the values, such as dividing the ionization energies and electron affinities by 230kj/mol) (b) Plot both sets of electronegativities against atomic number (use the same graph). (c) Which scale depends most consistently on position in the Periodic Table?arrow_forwardBelow is the SN2 reaction between 2-bromopropane and iodide (I). Draw the mechanism arrows in the first box to reflect electron movements. In both boxes, add lone pairs of electrons and nonzero formal charges. 4th attempt Feedback 3rd attempt Feedback 1 -Br H :Bri :Br: ili See Periodic Table See Hint ini See Periodic Table See Hintarrow_forwardWhen 4-chloro-1-butanol is placed in sodium hydride, a cyclization reaction occurs. 3rd attempt 2 HO NaH CI D Draw the curved arrow notation to form the intermediate. 4 2 H₂ See Periodic Table See Hint =arrow_forward
- Sketch, qualitatively, the potential energy curves of the N-N bond of N2H4, N2 and N3- graph. Explain why the energy at the minimum of each curve is not the same.arrow_forward(a) Show that the lattice energies are inversely proportional to the distance between ions in MX (M = alkali metal, X = halide ions) by plotting the lattice energies of KF, KCl, and KI against the internuclear distances, dMX. The lattice energies of KF, KCl, and KI are 826, 717, and 645 kJ/mol, respectively. Does the correlation obtained correlate well? You will need to use a standard graphing program to construct the graph (such as a spreadsheet program). It will generate an equation for the line and calculate a correlation coefficient. (b) Estimate the lattice energy of KBr from your graph. (c) Find an experimental value for the lattice energy of KBr in the literature, and compare this value with the one calculated in (b). Do they agree?arrow_forwardShow the curved arrow mechanism and both products for the reaction between methyl iodide and propoxide. 1st attempt NV H 10: H H 1 Add the missing curved arrow notation. H + See Periodic Tablearrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





