
Organic Chemistry
8th Edition
ISBN: 9781305580350
Author: William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
Chapter 11, Problem 11.18P
(a)
Interpretation Introduction
Interpretation:
The pair of chain propagation steps for the formation of ether hydroperoxide has to be accounted.
Concept Introduction:
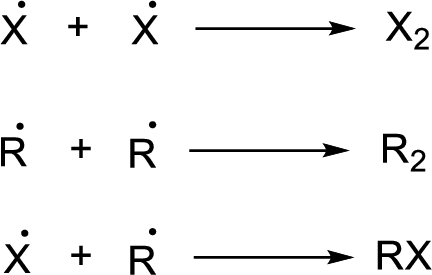
Radical chain reaction:
Initiation reaction: 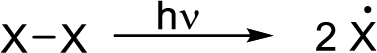
Chain propagation: 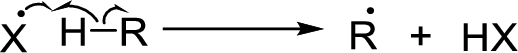
Chain termination:
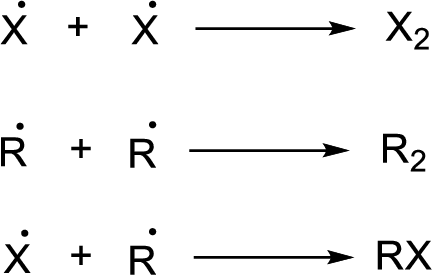
(b)
Interpretation Introduction
Interpretation:
The hydroperoxidation of ethers is regioselective; the given statement has to be accounted.
Concept Introduction:
Radical chain reaction:
Initiation reaction: 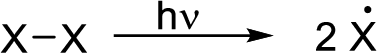
Chain propagation: 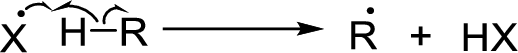
Chain termination:
Expert Solution & Answer
Trending nowThis is a popular solution!

Students have asked these similar questions
For each of the following reactions: Fill in the missing reactant, reagent, or product (s), indicating stereochemistry where appropriate using dashed and wedged bonds. If the reaction forms a racemic mixture, draw both structures in the box and write the word “racemic”.
1) Draw the correct chemical structure (using line-angle drawings / "line structures") from their
given IUPAC name:
a.
hept-3-yne
b. 5-bromo-1-fluoro-4-methylpent-2-yne
15. How many absorptions are expected in the H-NMR spectra of fee songs? Explain your were
a) CH,CH,CCH,CH,
O
CHUCH CHCH
Chapter 11 Solutions
Organic Chemistry
Ch. 11.2 - Write IUPAC and common names for these ethers. (a)...Ch. 11.3 - Arrange these compounds in order of increasing...Ch. 11.4 - Show how you might use the Williamson ether...Ch. 11.4 - Show how ethyl hexyl ether might be prepared by a...Ch. 11.5 - Account for the fact that treatment of tert-butyl...Ch. 11.5 - Draw structural formulas for the major products of...Ch. 11.6 - Prob. 11.7PCh. 11.8 - Draw the expected products of Sharpless...Ch. 11.9 - Prob. AQCh. 11.9 - Prob. BQ
Ch. 11.9 - Prob. CQCh. 11.9 - Prob. DQCh. 11 - Write names for these compounds. Where possible,...Ch. 11 - Prob. 11.11PCh. 11 - Each compound given in this problem is a common...Ch. 11 - Account for the fact that tetrahydrofuran (THF) is...Ch. 11 - Prob. 11.14PCh. 11 - Write equations to show a combination of reactants...Ch. 11 - Propose a mechanism for this reaction.Ch. 11 - Prob. 11.17PCh. 11 - Prob. 11.18PCh. 11 - Prob. 11.19PCh. 11 - Prob. 11.20PCh. 11 - Ethylene oxide is the starting material for the...Ch. 11 - Prob. 11.22PCh. 11 - Predict the structural formula of the major...Ch. 11 - The following equation shows the reaction of...Ch. 11 - Propose a mechanism to account for this...Ch. 11 - Acid-catalyzed hydrolysis of the following epoxide...Ch. 11 - Prob. 11.27PCh. 11 - Prob. 11.28PCh. 11 - Prob. 11.29PCh. 11 - Propose a mechanism for the following...Ch. 11 - Show reagents and experimental conditions to...Ch. 11 - Starting with cis-3-hexene, show how to prepare...Ch. 11 - Show reagents to convert cycloheptene to each of...Ch. 11 - Show reagents to convert bromocyclopentane to each...Ch. 11 - Prob. 11.35PCh. 11 - Starting with acetylene and ethylene oxide as the...Ch. 11 - Following are the steps in the industrial...Ch. 11 - Prob. 11.38PCh. 11 - Prob. 11.39PCh. 11 - Aldehydes and ketones react with one molecule of...Ch. 11 - Prob. 11.42PCh. 11 - Write the products of the following sequences of...Ch. 11 - Using your reaction roadmap as a guide, show how...Ch. 11 - Using your reaction roadmap as a guide, show how...Ch. 11 - Using your reaction roadmap as a guide, show how...Ch. 11 - During the synthesis of the antiasthmatic drug...Ch. 11 - Prob. 11.48P
Knowledge Booster
Similar questions
- Firefly luciferin exhibits three rings. Identify which of the rings are aromatic. Identify which lone pairs are involved in establishing aromaticity. The lone pairs are labeled A-D below.arrow_forwardWhat is the [OH⁻] of a 1.80 M solution of pyridine (C₅H₅N, Kb = 1.70 × 10⁻⁹)?arrow_forwardWhat is the percent ionization in a 0.260 M solution of formic acid (HCOOH) (Ka = 1.78 × 10⁻⁴)?arrow_forward
- Determine the pH of solution of HC3H5O2 By constructing an ICE table writing the equilibrium constant expression, and using this information to determine the pH. The Ka of HC3H5O2 is 1.3 x 10-5arrow_forwardDetermine if the following salt is neutral, acidic or basic. If acidic or basic, write the appropriate equilibrium equation for the acid or base that exists when the salt is dissolved in aqueous solution. If neutral, simply write only NR. Be sure to include the proper phases for all species within the reaction LiNO3arrow_forwardAn unknown weak acid with a concentration of 0.410 M has a pH of 5.600. What is the Ka of the weak acid?arrow_forward
- (racemic) 19.84 Using your reaction roadmaps as a guide, show how to convert 2-oxepanone and ethanol into 1-cyclopentenecarbaldehyde. You must use 2-oxepanone as the source of all carbon atoms in the target molecule. Show all reagents and all molecules synthesized along the way. & + EtOH H 2-Oxepanone 1-Cyclopentenecarbaldehydearrow_forwardR₂ R₁ R₁ a R Rg Nu R₂ Rg R₁ R R₁₂ R3 R R Nu enolate forming R₁ R B-Alkylated carbonyl species or amines Cyclic B-Ketoester R₁₁ HOB R R₁B R R₁₂ B-Hydroxy carbonyl R diester R2 R3 R₁ RB OR R₂ 0 aB-Unsaturated carbonyl NaOR Aldol HOR reaction 1) LDA 2) R-X 3) H₂O/H₂O ketone, aldehyde 1) 2°-amine 2) acid chloride 3) H₂O'/H₂O 0 O R₁ R₁ R R₁ R₁₂ Alkylated a-carbon R₁ H.C R₁ H.C Alkylated methyl ketone acetoacetic ester B-Ketoester ester R₁ HO R₂ R B-Dicarbonyl HO Alkylated carboxylic acid malonic ester Write the reagents required to bring about each reaction next to the arrows shown. Next, record any regiochemistry or stereochemistry considerations relevant to the reaction. You should also record any key aspects of the mechanism, such as forma- tion of an important intermediate, as a helpful reminder. You may want to keep track of all reactions that make carbon-carbon bonds, because these help you build large molecules from smaller fragments. This especially applies to the reactions in…arrow_forwardProvide the reasonable steps to achieve the following synthesis.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you

 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning


Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning