
Organic Chemistry
8th Edition
ISBN: 9781305580350
Author: William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Chapter 11.9, Problem AQ
Interpretation Introduction
Interpretation:
The stereochemical outcome of the reaction at boxed and circled carbons relative to the reactant has to be discussed.
Concept Introduction:
Stereochemistry: The spatial arrangement of atoms or groups present in compound.
Ring opening: Ring opening of epoxide takes place by acid-catalyzed hydrolysis of an epoxide.
Acid-catalyzed hydrolysis of an epoxide:
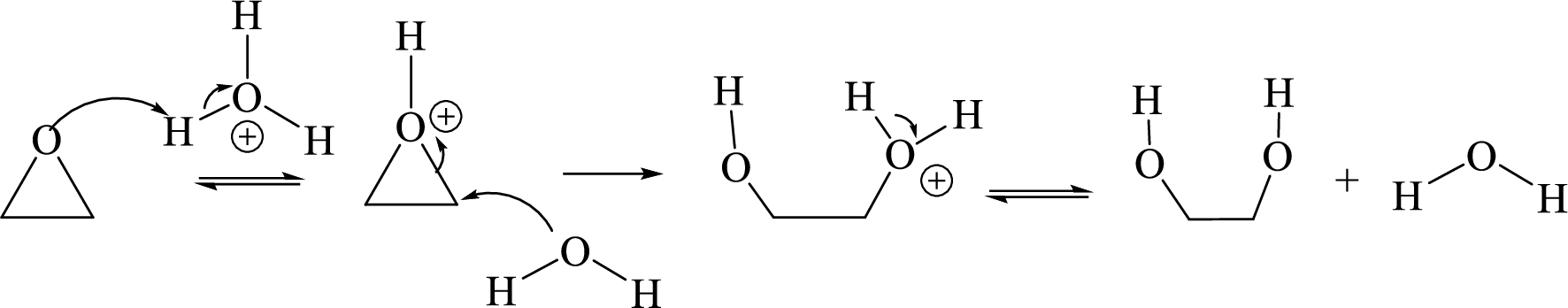
Expert Solution & Answer
Trending nowThis is a popular solution!

Students have asked these similar questions
Imagine each of the molecules shown below was found in an aqueous solution. Can you tell whether the solution is acidic, basic, or
neutral?
molecule
0=0
H3N-CH-C-o
HO
CH2
OH
The solution is...
O acidic
O basic
O neutral
O (unknown)
H₂N
acidic
O basic
O neutral
○ (unknown)
+
H3N
O
OH
O acidic
O basic
O neutral
O (unknown)
H2N-CH-C-O
CH3
O acidic
O basic
neutral
○ (unknown)
X
?
olo
HE
Recognizing ampli
Draw an a amino acid with a methyl (-CH3) side chain.
Explanation
Check
Click and drag to start drawing a
structure.
X
C
Write the systematic name of each organic molecule:
structure
name
×
HO
OH
☐
OH
CI
CI
O
CI
OH
OH
Chapter 11 Solutions
Organic Chemistry
Ch. 11.2 - Write IUPAC and common names for these ethers. (a)...Ch. 11.3 - Arrange these compounds in order of increasing...Ch. 11.4 - Show how you might use the Williamson ether...Ch. 11.4 - Show how ethyl hexyl ether might be prepared by a...Ch. 11.5 - Account for the fact that treatment of tert-butyl...Ch. 11.5 - Draw structural formulas for the major products of...Ch. 11.6 - Prob. 11.7PCh. 11.8 - Draw the expected products of Sharpless...Ch. 11.9 - Prob. AQCh. 11.9 - Prob. BQ
Ch. 11.9 - Prob. CQCh. 11.9 - Prob. DQCh. 11 - Write names for these compounds. Where possible,...Ch. 11 - Prob. 11.11PCh. 11 - Each compound given in this problem is a common...Ch. 11 - Account for the fact that tetrahydrofuran (THF) is...Ch. 11 - Prob. 11.14PCh. 11 - Write equations to show a combination of reactants...Ch. 11 - Propose a mechanism for this reaction.Ch. 11 - Prob. 11.17PCh. 11 - Prob. 11.18PCh. 11 - Prob. 11.19PCh. 11 - Prob. 11.20PCh. 11 - Ethylene oxide is the starting material for the...Ch. 11 - Prob. 11.22PCh. 11 - Predict the structural formula of the major...Ch. 11 - The following equation shows the reaction of...Ch. 11 - Propose a mechanism to account for this...Ch. 11 - Acid-catalyzed hydrolysis of the following epoxide...Ch. 11 - Prob. 11.27PCh. 11 - Prob. 11.28PCh. 11 - Prob. 11.29PCh. 11 - Propose a mechanism for the following...Ch. 11 - Show reagents and experimental conditions to...Ch. 11 - Starting with cis-3-hexene, show how to prepare...Ch. 11 - Show reagents to convert cycloheptene to each of...Ch. 11 - Show reagents to convert bromocyclopentane to each...Ch. 11 - Prob. 11.35PCh. 11 - Starting with acetylene and ethylene oxide as the...Ch. 11 - Following are the steps in the industrial...Ch. 11 - Prob. 11.38PCh. 11 - Prob. 11.39PCh. 11 - Aldehydes and ketones react with one molecule of...Ch. 11 - Prob. 11.42PCh. 11 - Write the products of the following sequences of...Ch. 11 - Using your reaction roadmap as a guide, show how...Ch. 11 - Using your reaction roadmap as a guide, show how...Ch. 11 - Using your reaction roadmap as a guide, show how...Ch. 11 - During the synthesis of the antiasthmatic drug...Ch. 11 - Prob. 11.48P
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- く Check the box under each a amino acid. If there are no a amino acids at all, check the "none of them" box under the table. Note for advanced students: don't assume every amino acid shown must be found in nature. COO H3N-C-H CH2 HO CH3 NH3 O CH3-CH CH2 OH Onone of them Explanation Check + H3N O 0. O OH + NH3 CH2 CH3-CH H2N C-COOH H O HIC + C=O H3N-C-O CH3- - CH CH2 OH Х 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center Accesarrow_forwardWrite the systematic name of each organic molecule: structure HO-C-CH2-CH3 O -OH CH3-CH2-CH2-CH2-CH2-C-OH CH3 CH3-CH-CH2-C-OH Explanation Check S namearrow_forwardtheres 2 productsarrow_forward
- Draw the major product of this solvolysis reaction. Ignore any inorganic byproducts. + CH3CH2OH Drawing Q Atoms, Bonds and Rings OCH2CH3 || OEt Charges OH 00-> | Undo Reset | Br Remove Done Drag To Pan +arrow_forwardDraw the major product of this SN1 reaction. Ignore any inorganic byproducts. CH3CO2Na CH3CO2H Drawing + Br Q Atoms, Bonds and Rings OAC Charges OH ОАс Na ဂ Br Undo Reset Remove Done Drag To Pan +arrow_forwardOrganic Functional Groups entifying positions labeled with Greek letters in acids and derivatives 1/5 ssible, replace an H atom on the a carbon of the molecule in the drawing area with a ce an H atom on the ẞ carbon with a hydroxyl group substituent. ne of the substituents can't be added for any reason, just don't add it. If neither substi er the drawing area. O H OH Oneither substituent can be added. Check D 1 Accessibility ado na witharrow_forward
- Differentiate between electrophilic and nucleophilic groups. Give examples.arrow_forwardAn aldehyde/ketone plus an alcohol gives a hemiacetal, and an excess of alcohol gives an acetal. The reaction is an equilibrium; in aldehydes, it's shifted to the right and in ketones, to the left. Explain.arrow_forwardDraw a Haworth projection or a common cyclic form of this monosaccharide: H- -OH H- OH H- -OH CH₂OHarrow_forward
- Answer the question in the first photoarrow_forwardGgggffg2258555426855 please don't use AI Calculate the positions at which the probability of a particle in a one-dimensional box is maximum if the particle is in the fifth energy level and in the eighth energy level.arrow_forwardExplain the concepts of hemiacetal and acetal.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning
