
Organic Chemistry
8th Edition
ISBN: 9781305580350
Author: William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Chapter 11, Problem 11.19P
Interpretation Introduction
Interpretation:
Synthesis of triethanolamine from ethylene oxide has to be proposed.
Concept introduction:
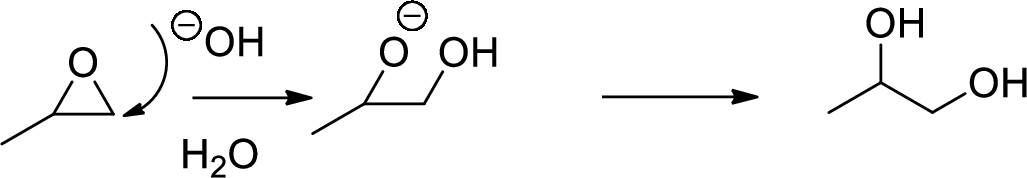
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Provide the reasonable steps to achieve the following synthesis.
Identify which compound is more acidic. Justify your choice.
Provide the reasonable steps to achieve the following synthesis.
Chapter 11 Solutions
Organic Chemistry
Ch. 11.2 - Write IUPAC and common names for these ethers. (a)...Ch. 11.3 - Arrange these compounds in order of increasing...Ch. 11.4 - Show how you might use the Williamson ether...Ch. 11.4 - Show how ethyl hexyl ether might be prepared by a...Ch. 11.5 - Account for the fact that treatment of tert-butyl...Ch. 11.5 - Draw structural formulas for the major products of...Ch. 11.6 - Prob. 11.7PCh. 11.8 - Draw the expected products of Sharpless...Ch. 11.9 - Prob. AQCh. 11.9 - Prob. BQ
Ch. 11.9 - Prob. CQCh. 11.9 - Prob. DQCh. 11 - Write names for these compounds. Where possible,...Ch. 11 - Prob. 11.11PCh. 11 - Each compound given in this problem is a common...Ch. 11 - Account for the fact that tetrahydrofuran (THF) is...Ch. 11 - Prob. 11.14PCh. 11 - Write equations to show a combination of reactants...Ch. 11 - Propose a mechanism for this reaction.Ch. 11 - Prob. 11.17PCh. 11 - Prob. 11.18PCh. 11 - Prob. 11.19PCh. 11 - Prob. 11.20PCh. 11 - Ethylene oxide is the starting material for the...Ch. 11 - Prob. 11.22PCh. 11 - Predict the structural formula of the major...Ch. 11 - The following equation shows the reaction of...Ch. 11 - Propose a mechanism to account for this...Ch. 11 - Acid-catalyzed hydrolysis of the following epoxide...Ch. 11 - Prob. 11.27PCh. 11 - Prob. 11.28PCh. 11 - Prob. 11.29PCh. 11 - Propose a mechanism for the following...Ch. 11 - Show reagents and experimental conditions to...Ch. 11 - Starting with cis-3-hexene, show how to prepare...Ch. 11 - Show reagents to convert cycloheptene to each of...Ch. 11 - Show reagents to convert bromocyclopentane to each...Ch. 11 - Prob. 11.35PCh. 11 - Starting with acetylene and ethylene oxide as the...Ch. 11 - Following are the steps in the industrial...Ch. 11 - Prob. 11.38PCh. 11 - Prob. 11.39PCh. 11 - Aldehydes and ketones react with one molecule of...Ch. 11 - Prob. 11.42PCh. 11 - Write the products of the following sequences of...Ch. 11 - Using your reaction roadmap as a guide, show how...Ch. 11 - Using your reaction roadmap as a guide, show how...Ch. 11 - Using your reaction roadmap as a guide, show how...Ch. 11 - During the synthesis of the antiasthmatic drug...Ch. 11 - Prob. 11.48P
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- When anisole is treated with excess bromine, the reaction gives a product which shows two singlets in 1H NMR. Draw the product.arrow_forward(ii) Draw a reasonable mechanism for the following reaction: CI NaOH heat OH (hint: SNAr Reaction) :arrow_forwardDraw the major product in each of the following reaction:arrow_forward
- Draw the mechanism for the following Friedel-Craft reaction. AlBr3 Brarrow_forward(a) Draw the structures of A and B in the following reaction. (i) NaNH2, NH3(1) A + B (ii) H3O+arrow_forwardFor the reaction 2 N2O5(g) → 4 NO2(g) + O2(g), the following mechanism has been proposed: N2O5 →> NO₂+ NO3_(K1) NO2 + NO3 → N2O5 (k-1) NO2 + NO3 → → NO2 + O2 + NO (K2) NO + N2O5- NO2 + NO2 + NO2 (K3) d[N₂O5] __2k‚k₂[N2O5] Indicate whether the following rate expression is acceptable: dt k₁₁+ k₂arrow_forward
- Consider the following decomposition reaction of N2O5(g): For the reaction 2 N2O5(g) → 4 NO2(g) + O2(g), the following mechanism has been proposed: N2O5 → NO2 + NO3 (K1) NO2 + NO3 → N2O5 (k-1) NO2 + NO3 → NO2 + O2 + NO (K2) NO + N2O5 → NO2 + NO2 + NO2 (K3) Indicate whether the following rate expression is acceptable: d[N2O5] = -k₁[N₂O₂] + K¸₁[NO₂][NO3] - K¸[NO₂]³ dtarrow_forwardIn a reaction of A + B to give C, another compound other than A, B or C may appear in the kinetic equation.arrow_forwardFor the reaction 2 N2O5(g) → 4 NO2(g) + O2(g), the following mechanism has been proposed: N2O5 →> NO₂+ NO3_(K1) NO2 + NO3 → N2O5 (k-1) NO2 + NO3 → → NO2 + O2 + NO (K2) NO + N2O5- NO2 + NO2 + NO2 (K3) d[N₂O5] __2k‚k₂[N2O5] Indicate whether the following rate expression is acceptable: dt k₁₁+ k₂arrow_forward
- Given the reaction R + Q → P, indicate the rate law with respect to R, with respect to P and with respect to P.arrow_forwardSteps and explanations. Also provide, if possible, ways to adress this kind of problems in general.arrow_forwardk₁ Given the reaction A B, indicate k-1 d[A] (A). the rate law with respect to A: (B). the rate law with respect to B: d[B] dt dtarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you

 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning


Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning