
Show reagents and experimental conditions to synthesize the following compounds from 1-propanol (any derivative of 1-propanol prepared in one part of this problem may be used for the synthesis of another part of the problem).
- (a) Propanal
- (b) Propanoic acid
- (c) Propene
- (d) 2-Propanol
- (e) 2-Bromopropane
- (f) 1-Chloropropane
- (g) 1,2-Dibromopropane
- (h) Propyne
- (i) 2-Propanone
- (j) 1-Chloro-2-propanol
- (k) Methyloxirane
- (l) Dipropyl ether
- (m) Isopropyl propyl ether
- (n) 1-Mercapto-2-propanol
- (o) 1-Amino-2-propanol
- (p) 1,2-Propanediol
(a)
Interpretation:
Conversion of Propanol to given desired product has to be shown.
Concept introduction:
Oxidation of primary alcohol to aldehyde: A Primary alcohol is oxidized to an aldehyde by PCC.

Explanation of Solution
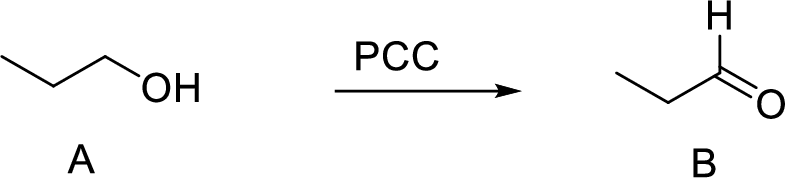
The oxidation of primary alcohol to aldehyde is performed using PCC gives rise to the desired aldehyde (Propanal) product as shown above.
(b)
Interpretation:
Conversion of Propanol to given desired product has to be shown.
Concept introduction:
Hydroboration-oxidation: Generally alkenes react with boron followed by hydrogen peroxide and in presence of base like sodium hydroxide it gives corresponding alcohol and it follows anti-Markovnikov rule.
Oxidation of primary alcohol to aldehyde: A Primary alcohol is oxidized to an aldehyde by PCC.

Explanation of Solution
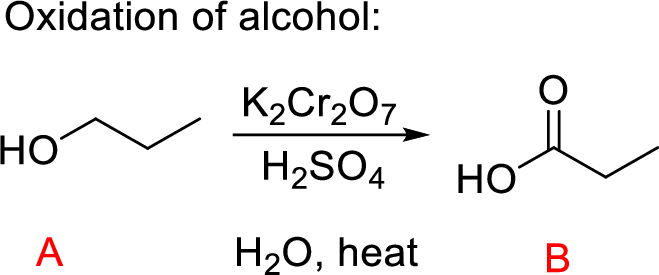
The oxidation of primary alcohol (A) to carboxylic acid (C) is performed using chromic acid on heating; the aldehyde with water in the solution g9ives hydrated aldehyde intermediate leads to the desired Propanoic acid product as shown above.
(c)
Interpretation:
Conversion of Propanol to given desired product has to be shown.
Concept introduction:
Acid-catalyzed dehydration of alcohols: An alcohol can be converted into an alkene by dehydration (with elimination of water from adjacent carbon). By heating alcohol with either 85% phosphoric acid or concentrated sulfuric acid. Primary alcohol require high temperature as high as
Ease of dehydration of alcohols:
The ease of acid-catalyzed dehydration of alcohols is in the order as follows,
Explanation of Solution
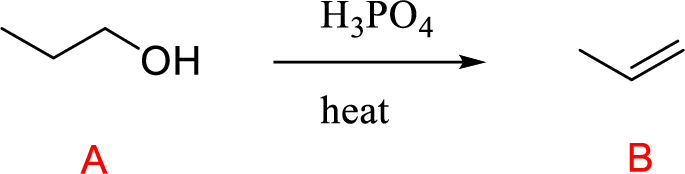
The alcohol (A) compound undergoes acid-catalyzed dehydration
Acid-catalyzed dehydration reaction of alcohol with
(d)
Interpretation:
Conversion of Propanol to given desired product has to be shown.
Concept introduction:
Acid-catalyzed dehydration of alcohols: An alcohol can be converted into an alkene by dehydration (with elimination of water from adjacent carbon). By heating alcohol with either 85% phosphoric acid or concentrated sulfuric acid. Primary alcohol require high temperature as high as
Ease of dehydration of alcohols:
The ease of acid-catalyzed dehydration of alcohols is in the order as follows,
Acid Catalyzed Hydration Reaction: The reaction involves breaking of pi bonds between carbon-carbon multiple bonds and addition of alcohol to more substituted position of carbon in the molecule.
First step is the acid donates proton to the alkene which leads to the formation of more stable carbo cation.
Then, the water is added to the given alkene through acid catalyzed reaction where the water gets added to the carbo cation finally, the removal of one proton from oxonium ion (oxygen with one positive charge) using water results in the formation of product.
Explanation of Solution
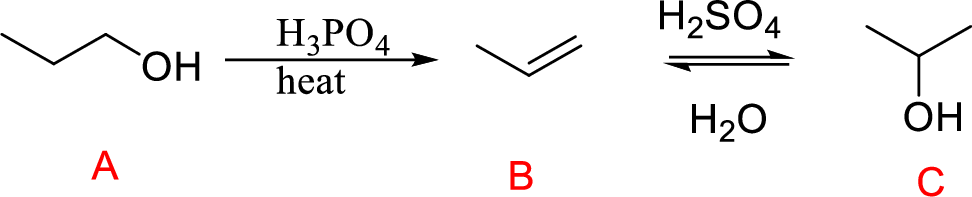
In the above reaction, A to B involves acid-catalyzed dehydration process; followed by acid-catalyzed hydration process (B to C).
Acid-catalyzed hydration:
Initially
Finally, the oxonium ion removes one proton gives the secondary alcohol as the major product.
(e)
Interpretation:
Conversion of Propanol to given desired product has to be shown.
Concept introduction:
Oxidation of secondary alcohol to aldehyde: A secondary alcohol is oxidized to a aldehyde by chromic acid. The mechanism involves initial formation of an alkyl chromate intermediate, followed by reaction with base to remove a proton, generating the carbonyl group of an aldehyde reducing the chromium (VI) to chromium (IV).
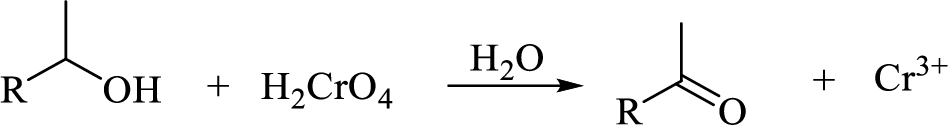
Explanation of Solution
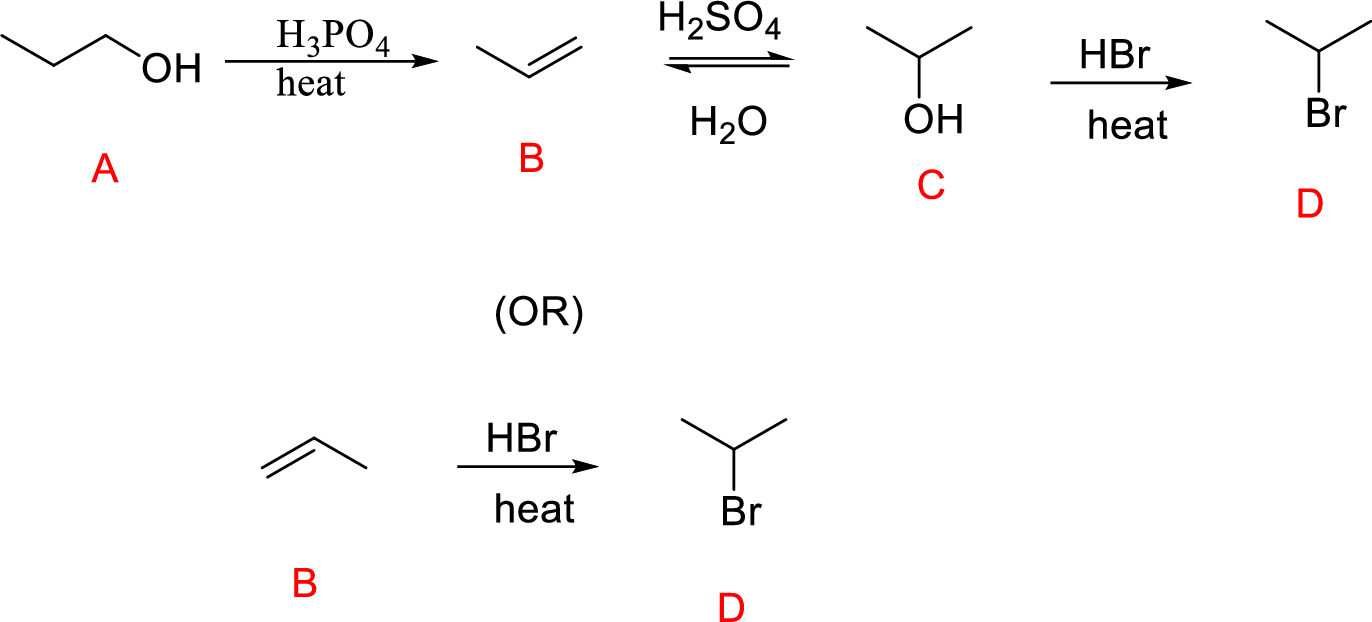
The Propanol undergoes acid-catalyzed dehydration; further acid-catalyzed hydration forming secondary alcohol (C), reaction of secondary alcohol with
(or)
Reaction of Compound (B) reacts with
(f)
Interpretation:
Conversion of Propanol to given desired product has to be shown.
Concept introduction:
Conversion of alcohol into haloalkane:
Conversion of primary and secondary alcohols to Chloroalkane is thionyl chloride. The reaction is carried out in the presence of base such as pyridine or trimethylamine.
The reaction of an alcohol with thionyl chloride is the formation of an alkyl Chlorosulfite that converts hydroxide ion (poor leaving group) into Chlorosulfite (good leaving group). Nucleophilic displacement of this leaving group gives product.
Leaving group: Leaving group can be any groups or atoms that get detached from either neutral or charged organic compounds. The stability of the leaving group is to stabilize the electron density that results from heterolysis cleavage of bond.
Explanation of Solution
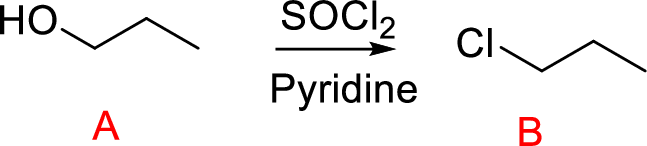
Reaction of alcohol (A) with
(g)
Interpretation:
Conversion of Propanol to given desired product has to be shown.
Concept introduction:
Acid-catalyzed dehydration of primary and secondary alcohols: Dehydration of primary and secondary alcohols is often accompanied by rearrangement process (shift of hydride or alkyl from
Addition of halogen across double bond: The reaction of alkene with halogen molecules undergoes electrophilic addition reaction forming 1,2-dihaloalkane compound.
Explanation of Solution
The reaction is shown below,
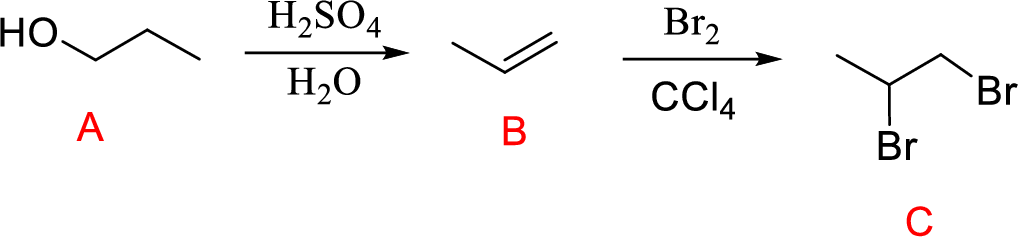
Acid-catalyzed dehydration reaction of alcohol with
Further reaction of alkene by addition of bromine across double bond gives 1,2-dibromopropane.
(h)
Interpretation:
Conversion of Propanol to given desired product has to be shown.
Concept introduction:
Acid-catalyzed dehydration of primary and secondary alcohols: Dehydration of primary and secondary alcohols is often accompanied by rearrangement process (shift of hydride or alkyl from
Addition of halogen across double bond: The reaction of alkene with halogen molecules undergoes electrophilic addition reaction forming 1,2-dihaloalkane compound.
Explanation of Solution
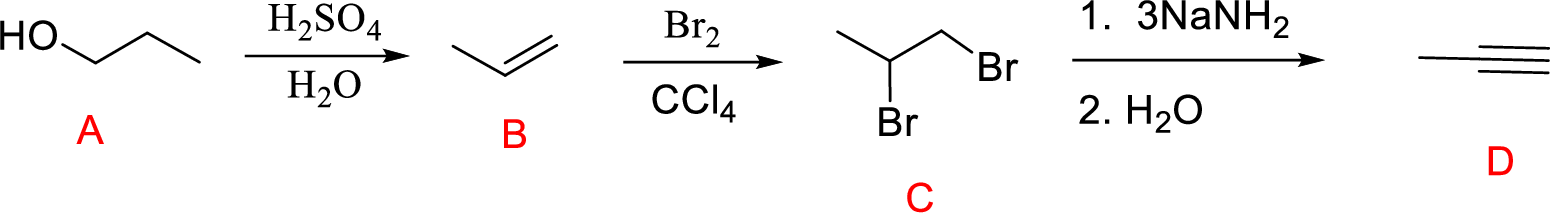
Acid-catalyzed dehydration reaction of alcohol with
Further reaction of alkene by addition of bromine across double bond gives 1,2-dibromopropane.
Addition of strong base leads to the elimination of two moles of
(i)
Interpretation:
Conversion of Propanol to given desired product has to be shown.
Concept introduction:
Acid-catalyzed dehydration of primary and secondary alcohols: Dehydration of primary and secondary alcohols is often accompanied by rearrangement process (shift of hydride or alkyl from
Explanation of Solution
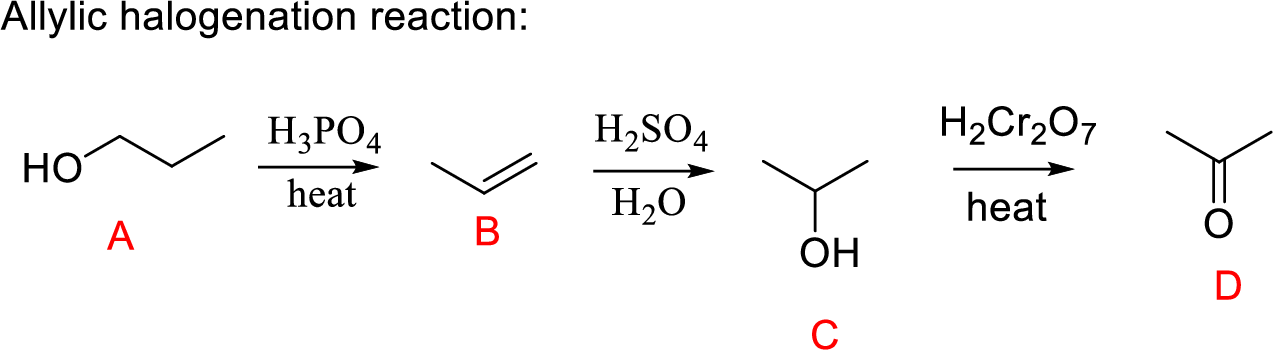
Propanol (A) undergoes dehydration followed by hydration gives secondary alcohol; the oxidation of secondary alcohol to ketone (D) the desired product.
(j)
Interpretation:
Conversion of Propanol to given desired product has to be shown.
Concept introduction:
Acid-catalyzed dehydration of primary and secondary alcohols: Dehydration of primary and secondary alcohols is often accompanied by rearrangement process (shift of hydride or alkyl from
Addition of halogen across double bond: The reaction of alkene with halogen molecules undergoes electrophilic addition reaction forming 1,2-dihaloalkane compound.
Explanation of Solution
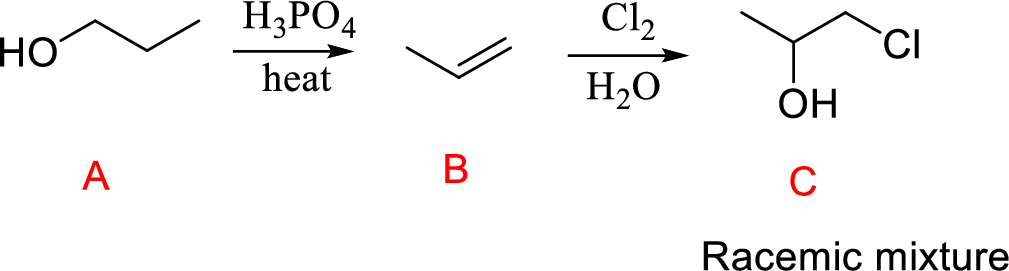
Propanol (A) reacts with phosphoric acid undergoes acid-catalyzed dehydration forming compound (B); further reaction with chlorine molecule leads to the compound (B); further reaction of compound (C).
(k)
Interpretation:
Conversion of Propanol to given desired product has to be shown.
Concept-Introduction:
Acid-catalyzed dehydration of primary and secondary alcohols: Dehydration of primary and secondary alcohols is often accompanied by rearrangement process (shift of hydride or alkyl from
Formation of epoxide: The alkene can be converted to epoxide when alkene is treated with MCPBA (m-Chloro per benzoic acid)

Explanation of Solution
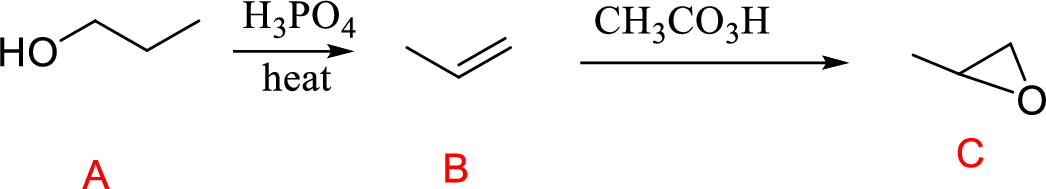
Propanol (A) with phosphoric acid on heating gives alkene compound (B) and further reaction with peroxyacid leads to the formation of epoxide compound (C).
(l)
Interpretation:
Conversion of Propanol to given desired product has to be shown.
Concept-Introduction:
Williamson ether synthesis: A general method for the synthesis of dialkyl ethers by a
Yield of ether becomes highest when the halide to be displaced is on primary carbon; yields are low in the displacement from secondary halides (because of
Explanation of Solution
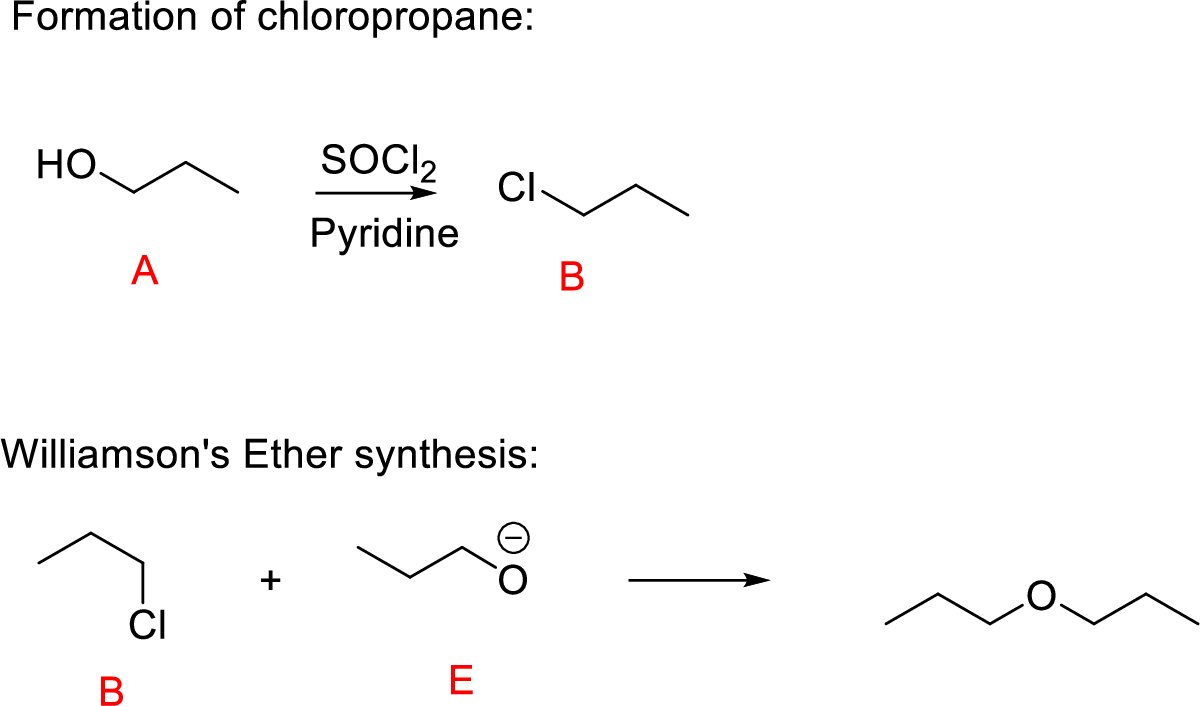
Propanol (A) reacts with
(m)
Interpretation:
Conversion of Propanol to given desired product has to be shown.
Concept-Introduction:
Williamson ether synthesis: A general method for the synthesis of dialkyl ethers by a
Yield of ether becomes highest when the halide to be displaced is on primary carbon; yields are low in the displacement from secondary halides (because of
Explanation of Solution
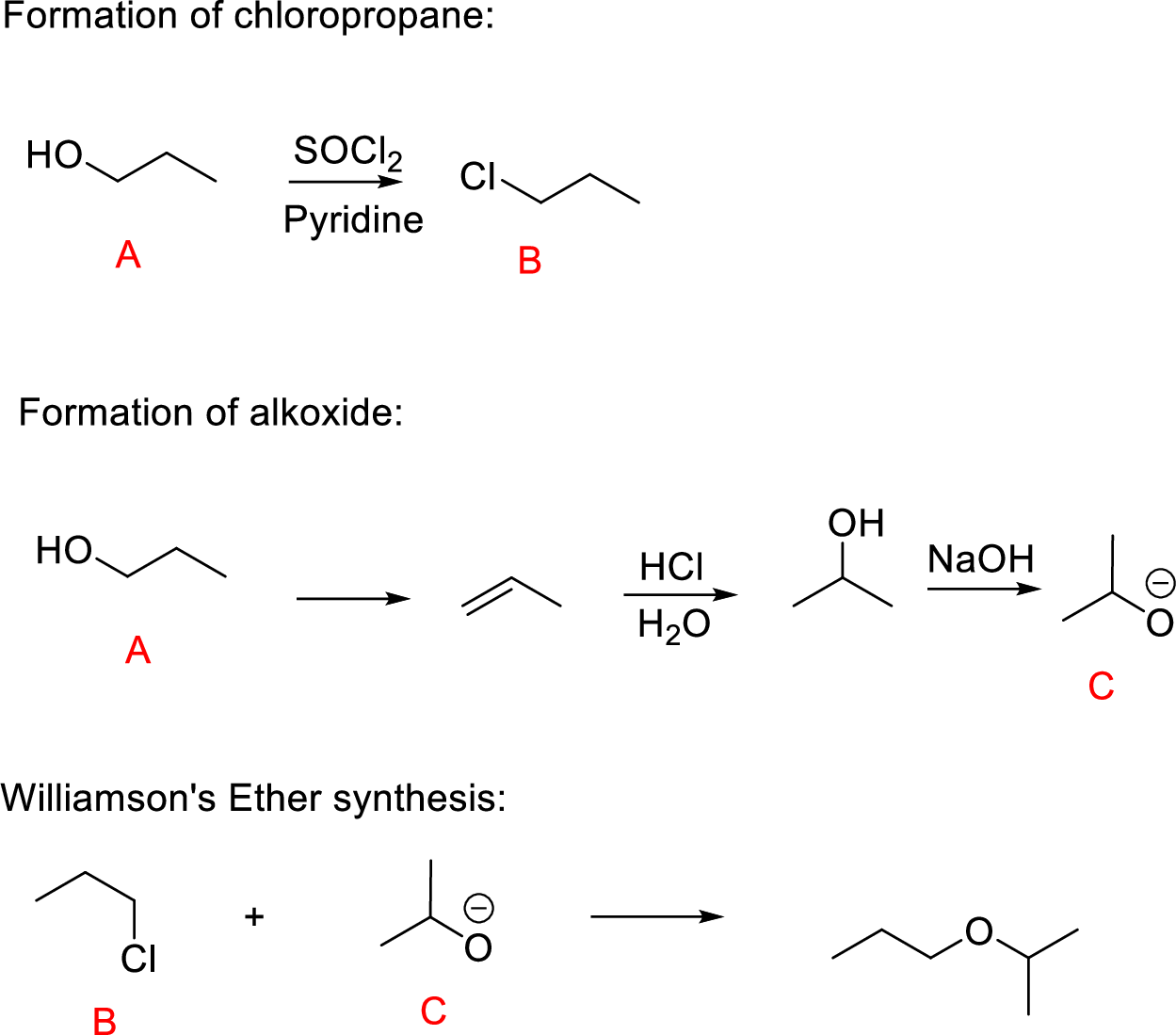
Propanol (A) reacts with
The reaction of compound (B) and compound (C) undergoes Williamson’s ether synthesis forming ether the desired product.
(n)
Interpretation:
Conversion of Propanol to given desired product has to be shown.
Concept-Introduction:
Acid-catalyzed dehydration of primary and secondary alcohols: Dehydration of primary and secondary alcohols is often accompanied by rearrangement process (shift of hydride or alkyl from
Formation of epoxide: The alkene can be converted to epoxide when alkene is treated with MCPBA (m-Chloro per benzoic acid)

Explanation of Solution
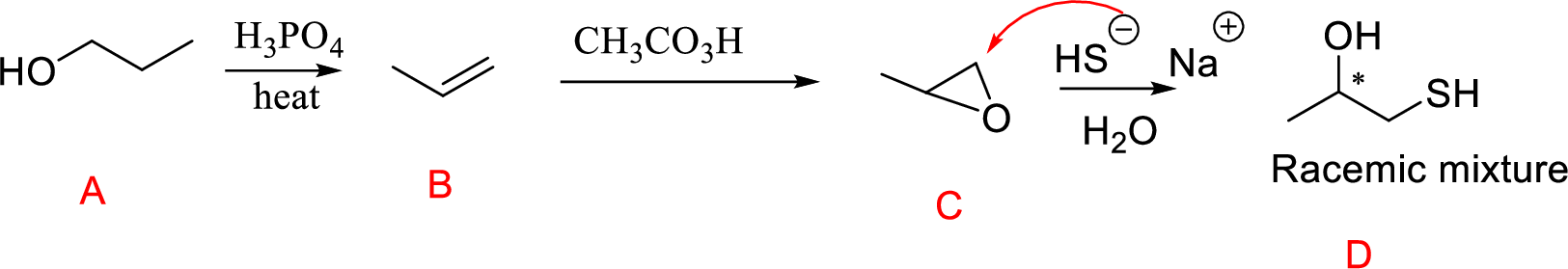
Propanol (A) with phosphoric acid on heating gives alkene compound (B) and further reaction with peroxyacid leads to the formation of epoxide compound (C).
The epoxide ring opening using the nucleophile
(o)
Interpretation:
Conversion of Propanol to given desired product has to be shown.
Concept Introduction:
Acid-catalyzed dehydration of primary and secondary alcohols: Dehydration of primary and secondary alcohols is often accompanied by rearrangement process (shift of hydride or alkyl from
Formation of epoxide: The alkene can be converted to epoxide when alkene is treated with MCPBA (m-Chloro per benzoic acid)

Explanation of Solution
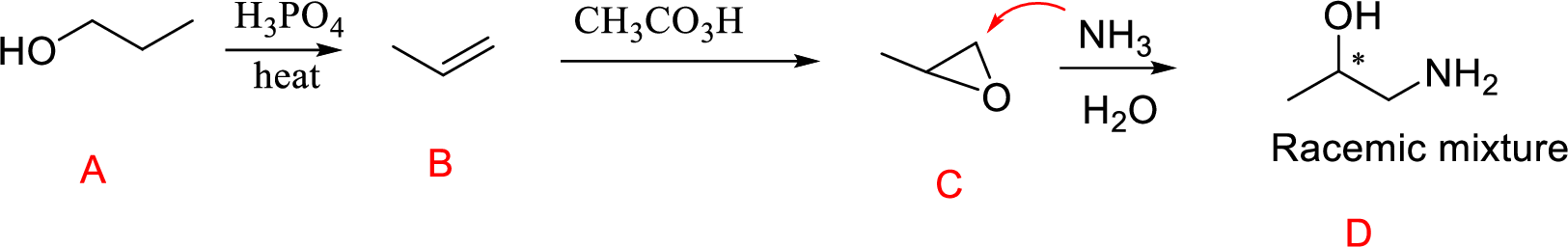
Propanol (A) with phosphoric acid on heating gives alkene compound (B) and further reaction with peroxyacid leads to the formation of epoxide compound (C).
The epoxide ring opening using the nucleophile
(p)
Interpretation:
Conversion of Propanol to given desired product has to be shown.
Concept introduction:
An alkene undergoes an oxidation reaction with Osmium tetra oxide and followed by hydrolysis to give a cis 1, 2 diol for example,
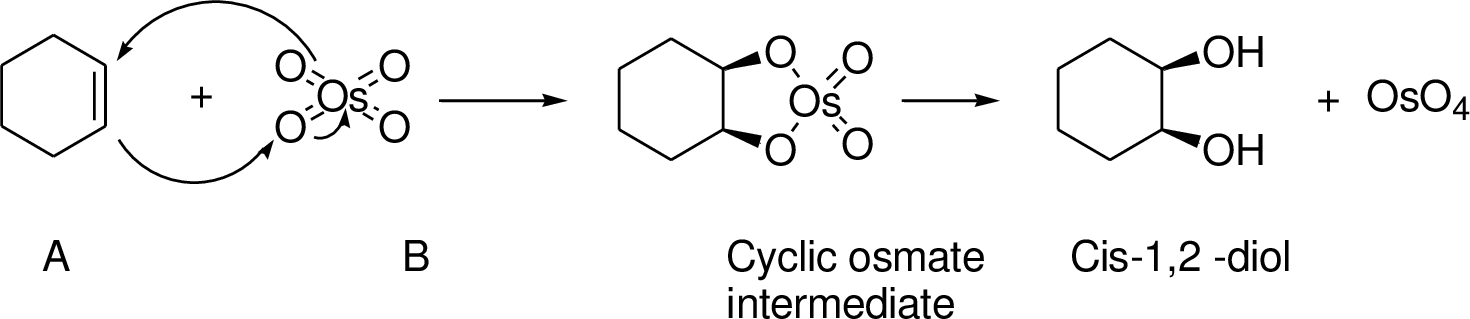
Explanation of Solution
The reaction is shown below,
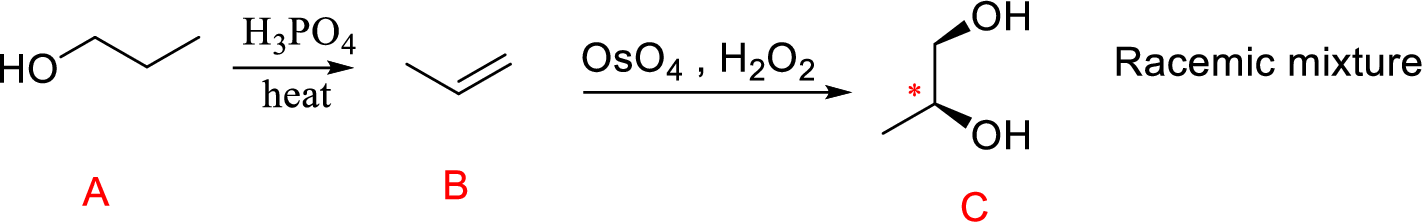
Acid-catalyzed dehydration gives the alkene compound (B) and the alkene (B) undergoes oxidation using osmium tetra oxide gives cyclic osmate intermediate, this intermediate further reaction with hydrogen peroxide hydrolysis that leads to product 1,2 diol (C). The racemic mixture obtained.
Want to see more full solutions like this?
Chapter 11 Solutions
Organic Chemistry
Additional Science Textbook Solutions
Laboratory Experiments in Microbiology (12th Edition) (What's New in Microbiology)
Biology: Life on Earth (11th Edition)
Organic Chemistry
Fundamentals Of Thermodynamics
Human Biology: Concepts and Current Issues (8th Edition)
- Please predict the products for each of the following reactions. Clearly show the regiochemistry (Markovnikov vs anti-Markovnikov) and stereochemistry (syn- vs anti- or both). If a mixture of enantiomers is formed, please draw all the enantiomers. cold KMnO4, NaOH 2. DMS 1. 03 CH3OH Br2 1. 03 2. (CH3)2S H₂ Pd or Pt (catalyst) HBr 18 19 20 1 HBr ROOR (peroxide) H₂O H₂SO4 HCI HI 17 16 6 15 MCPBA 1. BH3 THF 2. H₂O2, NaOH 1. OsO4 2. H₂O₂ 110 CH3CO₂H (peroxyacid) 1. MCPBA 2. H₂O* Br2 H₂O BH3 THF B12 EtOH Pd or Ni (catalyst) D₂ (deuterium) Bra A B C D H OH H OH OH H OH α α α OH H OH OH фон d H "Harrow_forwardBriefly indicate the models that describe the structure of the interface: Helmholtz-Perrin, Gouy-Chapman, Stern and Grahame models.arrow_forwardElectrochemistry. Briefly describe the Gibbs model and the Gibbs absorption equation.arrow_forward
- Briefly state the electrocapillary equation for ideally polarized electrodes.arrow_forwardWhat is surface excess according to the Gibbs model?arrow_forwardUsing Benzene as starting materid show how each of the Following molecules Contel Ve syntheswed CHI 9. b -50311 с CHY 503H Ночто d. อ •NOV e 11-0-650 NO2arrow_forward
- The molecule PYRIDINE, 6th electrons and is therefore aromatre and is Assigned the Following structure contering Since aromatk moleculoy undergo electrophilic anomatic substitution, Pyridine shodd undergo The Following reaction + HNO3 12504 a. write all of the possible Mononitration Products that could Result From this reaction 18. Bared upon the reaction mechanison determime which of these producty would be the major Product of the hegetionarrow_forwarda. Explain Why electron withdrawing groups tend to be meta-Directors. Your answer Should lyclude all apropriate. Resonance contributing Structures fo. Explain why -ll is an outho -tura drccton even though chlorine has a very High Electronegativityarrow_forward9. Write Me product as well as the reaction Mechanism For each of the Following Vanctions +H₂504 4.50+ T C. +212 Fellz 237 b. Praw the potential energy Diagrams For each OF Mese Rauctions and account For any differences that appear in the two potential Puergy Diagrams which of here two reactions 19 Found to be Reversable, Rationalice your answer based upon the venation mechanisms and the potential energy diagrams.arrow_forward
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning Macroscale and Microscale Organic ExperimentsChemistryISBN:9781305577190Author:Kenneth L. Williamson, Katherine M. MastersPublisher:Brooks Cole
Macroscale and Microscale Organic ExperimentsChemistryISBN:9781305577190Author:Kenneth L. Williamson, Katherine M. MastersPublisher:Brooks Cole

