
In a solution at a constant H+ concentration, iodide ions react with hydrogen peroxide to produce iodine.
Â
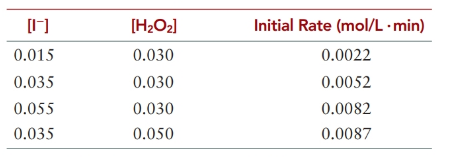
(a) What is rate expression for the reaction?
(b) Calculate k
(c) What is the rate of the reaction when 25.0 mL of a 0.100 M solution of KI is added to 25.0 mL of a 10.0% by mass solution of
Trending nowThis is a popular solution!

Chapter 11 Solutions
Chemistry: Principles and Reactions
- Don't used hand raitingarrow_forwardQuizzes - Gen Organic & Biological Che... ☆ myd21.lcc.edu + O G screenshot on mac - Google Search savings hulu youtube google disney+ HBO zlib Homework Hel...s | bartleby cell bio book Yuzu Reader: Chemistry G periodic table - Google Search b Home | bartleby 0:33:26 remaining CHEM 120 Chapter 5_Quiz 3 Page 1: 1 > 2 > 3 > 6 ¦ 5 > 4 > 7 ¦ 1 1 10 8 ¦ 9 a ¦ -- Quiz Information silicon-27 A doctor gives a patient 0.01 mC i of beta radiation. How many beta particles would the patient receive in I minute? (1 Ci = 3.7 x 10 10 d/s) Question 5 (1 point) Saved Listen 2.22 x 107 222 x 108 3.7 x 108 2.22 x 108 none of the above Question 6 (1 point) Listen The recommended dosage of 1-131 for a test is 4.2 μCi per kg of body mass. How many millicuries should be given to a 55 kg patient? (1 mCi = 1000 μСi)? 230 mCiarrow_forwardDon't used hand raiting and don't used Ai solutionarrow_forward
- Don't used hand raiting and don't used Ai solutionarrow_forwardQ3: Arrange each group of compounds from fastest SN2 reaction rate to slowest SN2 reaction rate. CI Cl H3C-Cl CI a) A B C D Br Br b) A B C Br H3C-Br Darrow_forwardQ4: Rank the relative nucleophilicity of halide ions in water solution and DMF solution, respectively. F CI Br | Q5: Determine which of the substrates will and will not react with NaSCH3 in an SN2 reaction to have a reasonable yield of product. NH2 Br Br Br .OH Brarrow_forward
- Classify each molecule as optically active or inactive. Determine the configuration at each H соон Chirality center OH 애 He OH H3C Ноос H H COOH A K B.arrow_forwardQ1: Rank the relative nucleophilicity of the following species in ethanol. CH3O¯, CH3OH, CH3COO, CH3COOH, CH3S Q2: Group these solvents into either protic solvents or aprotic solvents. Acetonitrile (CH3CN), H₂O, Acetic acid (CH3COOH), Acetone (CH3COCH3), CH3CH2OH, DMSO (CH3SOCH3), DMF (HCON(CH3)2), CH3OHarrow_forwardDon't used hand raiting and don't used Ai solutionarrow_forward
- 10. The main product of the following reaction is [1.1:4',1"-terphenyl]-2'-yl(1h-pyrazol-4- yl)methanone Ph N-H Pharrow_forwardDraw the Fischer projection for a D-aldo-pentose. (aldehyde pentose). How many total stereoisomers are there? Name the sugar you drew. Draw the Fischer projection for a L-keto-hexose. (ketone pentose). How many total stereoisomers are there? Draw the enantiomer.arrow_forwardDraw a structure using wedges and dashes for the following compound: H- Et OH HO- H H- Me OHarrow_forward
 Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning
Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning





