
Hydrogen bromide is a highly reactive and corrosive gas used mainly as a catalyst for organic reactions. It is produced by reacting hydrogen and bromine gases together.
Â
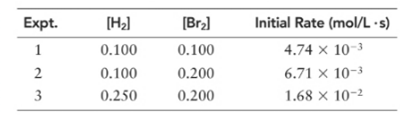
(a) What is the order of the reaction with respect to hydrogen, bromine, and overall?
(b) Write the rate expression of the reaction.
(c) Calculate k for the reaction. What are the units for k?
(d) When
(a)
Interpretation:
To determine the order of reaction with respect to BF3, NH3 and overall for the following reaction:
Concept introduction:
Rate of a chemical reaction: It tells us about the speed at which the reactants are converted into products.
Mathematically, rate of reaction is directly proportional to the product of concentration of each reactant raised to the power equal to their respective stoichiometric coefficients.
Let’s say we have a reaction:
Answer to Problem 27QAP
Order of given reaction:
With respect to H2 =1
With respect to Br2 =
Overall =
Explanation of Solution
Given information:
Here the chemical reaction is:

Let’s assume the reaction to be ‘t’ order with respect to H2 and ‘y’ order with respect to Br2.
Then, rate law for experiment 1 in above reaction will be;
And, rate law for experiment 3 in above reaction will be;
Divide (1) by (2) to get value of ‘t’.
Thus, order with respect to Br2 is
Now, rate law for experiment 4 in above reaction will be;
Divide (2) by (3) to get value of ‘y’.
Thus, order with respect to H2 is 1.
And the order of reaction will be:
Thus, overall order of reaction is 2.
(b)
Interpretation:
To write the rate expression for the given reaction.
Concept introduction:
Rate of a chemical reaction: It tells us about the speed at which the reactants are converted into products.
Mathematically, rate of reaction is directly proportional to the product of concentration of each reactant raised to the power equal to their respective stoichiometric coefficients.
Let’s say we have a reaction:
Answer to Problem 27QAP
Rate law expression for above reaction will be;
Explanation of Solution
Here the chemical reaction is:
Order of reaction with respect to H2 = 2
Order of reaction with respect to Br2 =
Let the rate constant be ‘k’.
Then, rate law expression for above reaction will be;
(c)
Interpretation:
To determine the rate constant and its unit for the given reaction.
Concept introduction:
Rate of a chemical reaction: It tells us about the speed at which the reactants are converted into products.
Mathematically, rate of reaction is directly proportional to the product of concentration of each reactant raised to the power equal to their respective stoichiometric coefficients.
Let’s say we have a reaction:
Answer to Problem 27QAP
Rate constant is
Unit of rate constant is
Explanation of Solution
Here the chemical reaction is:
Writing rate law for experiment 1 in above reaction will be;
Hence, the rate constant is
And unit of rate constant is
(d)
Interpretation:
To determine the rate of reaction at given concentration of reactants.
Concept introduction:
Rate of a chemical reaction: It tells us about the speed at which the reactants are converted into products.
Mathematically, rate of reaction is directly proportional to the product of concentration of each reactant raised to the power equal to their respective stoichiometric coefficients.
Let’s say we have a reaction:
Answer to Problem 27QAP
Rate of reaction for given reaction at given conditions is
Explanation of Solution
Here the chemical reaction is:
Rate law expression for above reaction:
Here we have:
[H2 ]=0.421 M
[Br2 ] = 0.215 M
Rate constant =
Plugging values in rate law as:
Hence, the rate of reaction is
Want to see more full solutions like this?
Chapter 11 Solutions
Chemistry: Principles and Reactions
- Dr. Mendel asked his BIOL 260 class what their height was and what their parent's heights were. He plotted that data in the graph below to determine if height was a heritable trait. A. Is height a heritable trait? If yes, what is the heritability value? (2 pts) B. If the phenotypic variation is 30, what is the variation due to additive alleles? (2 pts) Offspring Height (Inches) 75 67.5 60 52.5 y = 0.9264x + 4.8519 55 60 65 MidParent Height (Inches) 70 75 12pt v V Paragraph B IUA > AT2 v Varrow_forwardExperiment: Each team will be provided with 5g of a mixture of acetanilide and salicylic acid. You will divide it into three 1.5 g portions in separate 125 mL Erlenmeyer flasks savıng some for melting point analysis. Dissolve the mixture in each flask in ~60mL of DI water by heating to boiling on a hotplate. Take the flasks off the hotplate once you have a clear solution and let them stand on the bench top for 5 mins and then allow them to cool as described below. Sample A-Let the first sample cool slowly to room temperature by letting it stand on your lab bench, with occasional stirring to promote crystallization. Sample B-Cool the second sample 1n a tap-water bath to 10-15 °C Sample C-Cool the third sample in an ice-bath to 0-2 °C Results: weight after recrystalization and melting point temp. A=0.624g,102-115° B=0.765g, 80-105° C=1.135g, 77-108 What is the percent yield of A,B, and C.arrow_forwardRel. Intensity Q 1. Which one of the following is true of the compound whose mass spectrum is shown here? Explain how you decided. 100 a) It contains chlorine. b) It contains bromine. c) It contains neither chlorine nor bromine. 80- 60- 40- 20- 0.0 0.0 TT 40 80 120 160 m/z 2. Using the Table of IR Absorptions how could you distinguish between these two compounds in the IR? What absorbance would one compound have that the other compound does not? HO CIarrow_forward
- Illustrate reaction mechanisms of alkenes with water in the presence of H2SO4, detailing each step of the process. Please show steps of processing. Please do both, I will thumb up for sure #1 #3arrow_forwardDraw the following molecule: (Z)-1-chloro-1-butenearrow_forwardIdentify the molecule as having a(n) E, Z, cis, or trans configuration. CH3 H₁₂C ○ E ○ z ○ cis transarrow_forward
- Identify the molecule as having a(n) E, Z, cis, or trans configuration. H₂C- CH3 О Е ○ cis ○ transarrow_forwardThe decomposition of dinitrogen pentoxide according to the equation: 50°C 2 N2O5(g) 4 NO2(g) + O2(g) follows first-order kinetics with a rate constant of 0.0065 s-1. If the initial concentration of N2O5 is 0.275 M, determine: the final concentration of N2O5 after 180 seconds. ...arrow_forwardDon't used hand raitingarrow_forward
- CS2(g) →CS(g) + S(g) The rate law is Rate = k[CS2] where k = 1.6 × 10−6 s−¹. S What is the concentration of CS2 after 5 hours if the initial concentration is 0.25 M?arrow_forwardCS2(g) → CS(g) + S(g) The rate law is Rate = k [CS2] where k = 1.6 × 10-6 s−1. S Calculate the half-life.arrow_forwardThe following is a first order reaction where the rate constant, k, is 6.29 x 10-3 min-*** What is the half-life? C2H4 C2H2 + H2arrow_forward
 Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning





