
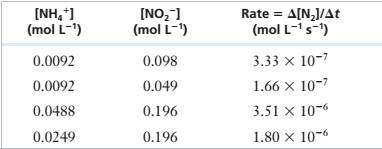
The following experimental data were obtained for the reaction of \'I14* and NOf in acidic solution.
NH/(aq) + NO2-(aq) — N;(g) + 2 H,O(f)
| INH/I (mol L1) | [NO21 (mol L-1, | Rate = A[NJ/At (mol L-1 s’) |
| 0.0092 | 0.098 | 3.33 X IO"7 |
| 0.0092 | 0.049 | 1.66 X 10‘7 |
| 0.0488 | 0.196 | 3.51 X 10"6 |
| 0.0249 | 0.196 | 1.80 X 10-6 |
Determine the rate law for this reaction and calculate the rate constant.
Interpretation: Given the experimental data obtained for a reaction, determine the rate law for the reaction and the value of rate constant
Concept Introduction: Orders of reaction are constantly determined by doing experiments. Consequently without experimental information, we can't conclude anything about the order of a reaction just by having a look at the equation for the reaction. By doing experiments involving a reaction between A and B, the rate of the reaction is identified to be related to the concentrations of A and B as follows:
This is the Rate Equation.
Where,
Rate is in the units of mol dm-3s-1
k is the rate constant
A, B- concentrations in mol dm-3
a - Order of reaction with respect to A
b- Order of reaction with respect to B
Answer to Problem 11.33PAE
Solution: The rate law of the reaction is
Explanation of Solution
Given information: Reaction of
Experimental Data
Step 1: For the reaction:
The rate law can be determined using the rate equation as follows:
Where,
a= Order of the reaction with respect to
b= Order of the reaction with respect to
Step 2: From the first and second rows of the given experimental data,
Step 3: Divide (1) by (2), we get
Step 4: From the third and fourth rows of the given experimental data,
Step 5: Divide (3) by (4), we get
Step 6: Rate Equation = >
To determine k, pick equation (1)
It does not make a difference what the number of reactants there are. The concentration of every reactant will be present in the rate equation, raised to some power. These powers resemble the individual orders with respect to each reactant. The sum of these powers results in the overall order of the reaction. The rate constant will be a constant value for a given reaction only if the concentration of the reactants is changed without changing any other factors.
Want to see more full solutions like this?
Chapter 11 Solutions
Chemistry for Engineering Students
- Calculate activation energy (Ea) from the following kinetic data: Temp (oC) Time (s) 23.0 180. 32.1 131 40.0 101 51.8 86.0 Group of answer choices 0.0269 kJ/mole 2610 kJ/mole 27.6 kJ/mole 0.215 kJ/mole 20.8 kJ/molearrow_forwardCalculate activation energy (Ea) from the following kinetic data: Temp (oC) Time (s) 23.0 180. 32.1 131 40.0 101 51.8 86.0 choices: 0.0269 kJ/mole 2610 kJ/mole 27.6 kJ/mole 0.215 kJ/mole 20.8 kJ/molearrow_forwardCalculate activation energy (Ea) from the following kinetic data: Temp (oC) Time (s) 23.0 180. 32.1 131 40.0 101 51.8 86.0arrow_forward
- Please solvearrow_forwardRank the compounds in each group below according to their reactivity toward electrophilic aromatic substitution (most reactive = 1; least reactive = 3). Place the number corresponding to the compounds' relative reactivity in the blank below the compound. a. CH₂F CH3 F b. At what position, and on what ring, is bromination of phenyl benzoate expected to occur? Explain your answer. :0: C-O phenyl benzoate 6.Consider the reaction below to answer the following questions. A B C NO₂ FeBr3 + Br₂ D a. The nucleophile in the reaction is: BODADES b. The Lewis acid catalyst in the reaction is: C. This reaction proceeds d. Draw the structure of product D. (faster or slower) than benzene.arrow_forwardPart 2. A solution of 6.00g of substance B in 100.0mL of aqueous solution is in equilibrium, at room temperature, wl a solution of B in diethyl ether (ethoxyethane) containing 25.0 g of B in 50.0 mL 9) what is the distribution coefficient of substance B b) what is the mass of B extracted by shaking 200 ml of an aqueous solution containing 10g of B with call at room temp): i) 100 mL of diethyl ether ii) 50ml of diethyl ether twice iii) 25ml of diethyl ether four timesarrow_forward
- - Rank the following groups of compounds from most acidic (1) to least acidic (4). Place the number corresponding to the compound's relative rank in the blank below the structure. a. NO₂ NO₂ CH2CH2CH2CH2OH CH3 CH3CH2CHOH CH3CH2CH2CH2OH NO₂ CH3CHCH2CH2OH b. OH OH CH₂OH CO₂H HC CN CN CNarrow_forwardGive the major organic product(s) of the following reactions or sequences of reactions. Show all relevant stereochemistry a. H MgBr 1. ether 2. H₂O* 4 COH b. 1. LIAIH, ether 2. H₂O Choose the best reagent(s) for carrying out the following conversions from the list provided below. Place the letter of the best choice in the blank to the left of the conversion. Reagents may be used more than once. a. 1. CH3MgBr, ether 2. H3O+ NaOH b. 1. PBr3 2. C. 2. 1. (CH3)3SiCl, (CH3CH2)3N CH3MgBr, ether 3. H₂O*+ 2. H3O+ e. 1. p-TosCl, pyridine f. نها g. 2. NaOH CrO3, H₂SO4, H₂O 1. NaBH4, ethanol 2. H30* h. PCC, CH2Cl2 Ovoldo-6 a. b. OH OH H OH O any organicarrow_forwardDetermine the rate law for sodium thiosulfate from the following data: [Na2S2O3] Time (s) 0.0318 230. 0.0636 57.5arrow_forward
 Physical ChemistryChemistryISBN:9781133958437Author:Ball, David W. (david Warren), BAER, TomasPublisher:Wadsworth Cengage Learning,
Physical ChemistryChemistryISBN:9781133958437Author:Ball, David W. (david Warren), BAER, TomasPublisher:Wadsworth Cengage Learning, Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning

