
Organic Chemistry
9th Edition
ISBN: 9781305080485
Author: John E. McMurry
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 10.SE, Problem 18MP
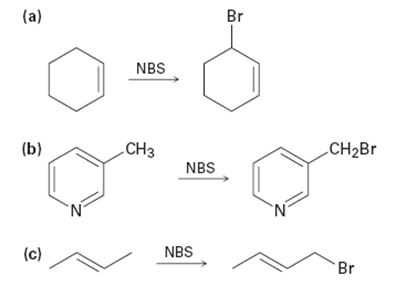
Draw the electron-pushing mechanism for the propagation steps of the allylic bromination reactions below. You may omit NBS in your mechanism, and use Br ∙ and Br2.
Expert Solution & Answer
Trending nowThis is a popular solution!

Students have asked these similar questions
Please correct answer and don't used hand raiting
9. The following reaction, which proceeds via the SN1/E1 mechanisms, gives three alkene products (A, B,
C) as well as an ether (D). (a) Show how each product arises mechanistically. (b) For the alkenes,
determine the major product and justify your answer. (c) What clues in the reaction as shown suggest
that this reaction does not go by the SN2/E2 mechanism route?
(CH3)2CH-CH-CH3 CH3OH
1
Bl
CH3OH ⑧· (CH3)2 CH-CH=CH2
heat
H
⑥③ (CH3)2 C = C = CH3
© СнЗ-С-Снаснз
сна
(CH 3 ) 2 C H G H CH 3
оснз
Please Don't used hand raiting
Chapter 10 Solutions
Organic Chemistry
Ch. 10.1 - Prob. 1PCh. 10.1 - Draw structures corresponding to the following...Ch. 10.2 - Prob. 3PCh. 10.2 - Taking the relative reactivities of 1°, 2°, and...Ch. 10.4 - Prob. 5PCh. 10.4 - The major product of the reaction of...Ch. 10.4 - Prob. 7PCh. 10.5 - Prob. 8PCh. 10.6 - Prob. 9PCh. 10.6 - How might you replace a halogen substituent by a...
Ch. 10.7 - How would you carry out the following...Ch. 10.8 - Rank both sets of compounds in order of increasing...Ch. 10.8 - Tell whether each of the following reactions is an...Ch. 10.SE - Prob. 14VCCh. 10.SE - Prob. 15VCCh. 10.SE - Prob. 16VCCh. 10.SE - Draw the electron-pushing mechanism for each...Ch. 10.SE - Draw the electron-pushing mechanism for the...Ch. 10.SE - The formation of Br2 from NBS first involves the...Ch. 10.SE - In light of the fact that tertiary alkyl halides...Ch. 10.SE - Alkyl halides can be reduced to alkanes by a...Ch. 10.SE - Name the following alkyl halides:Ch. 10.SE - Prob. 23APCh. 10.SE - Draw and name all of the monochlorination products...Ch. 10.SE - How would you prepare the following compounds,...Ch. 10.SE - Prob. 26APCh. 10.SE - A chemist requires a large amount of...Ch. 10.SE - What product(s) would you expect from the reaction...Ch. 10.SE - What product(s) would you expect from the reaction...Ch. 10.SE - What product would you expect from the reaction of...Ch. 10.SE - Rank the compounds in each of the following series...Ch. 10.SE - Which of the following compounds have the same...Ch. 10.SE - Tell whether each of the following reactions is an...Ch. 10.SE - Prob. 34APCh. 10.SE - Alkylbenzenes such as toluene (methylbenzene)...Ch. 10.SE - Prob. 36APCh. 10.SE - Prob. 37APCh. 10.SE - Prob. 38APCh. 10.SE - Prob. 39APCh. 10.SE - Prob. 40APCh. 10.SE - The syntheses shown here are unlikely to occur as...Ch. 10.SE - Why do you suppose its not possible to prepare a...Ch. 10.SE - Prob. 43APCh. 10.SE - Identify the reagents a–c in the following...Ch. 10.SE - Prob. 45APCh. 10.SE - Prob. 46APCh. 10.SE - Prob. 47APCh. 10.SE - The relative rate of radical bromination is...Ch. 10.SE - Prob. 49APCh. 10.SE - Predict the product and provide the entire...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- 7. For the following structure: ← Draw structure as is - NO BI H H Fisher projections (a) Assign R/S configuration at all chiral centers (show all work). Label the chiral centers with an asterisk (*). (b) Draw an enantiomer and diastereomer of the above structure and assign R/S configuration at all chiral centers (again, show all work). (c) On the basis of the R/S system, justify your designation of the structures as being enantiomeric or diastereomeric to the original structure.arrow_forwardDon't used Ai solutionarrow_forward1. For the following reactions, predict the major product. Show stereochemistry where appropriate. неу b) 7 HBr XV ROOR H₂504 c) N/ H20 H+2 d) ~ Pt c) f. MCPBA -> сна сла (solvent) (1)BH 3-THE (3) Надрон B177 H20 9)arrow_forward
- For the following reactions, predict the major product. Show stereochemistry where approarrow_forwardHow is Talu home quer in Org. Chemistry propose a 3-butanal prepared from ketone? complete reaction for this, (to start from the guignand Meagent. ②what pocubble products could be produced from the reaction of : CA₂ CH₂ CH₂ dil H.504 A CH3 1 OBCH₂OH Naz Cr₂ 07 12504 NazCD 4 CH3CH2 07 AzS04 H3C H3C CH3-C - C - Atz но но + H, CH3 07 > ⑦Colts C614501 + (215) 504 кон 4arrow_forwardRank the following compounds most to least acidic: a) О OH 요애 OH .OH flow flow О F F F F OH F b) Ha EN-Ha CI Ha F F CI Haarrow_forward
- a) b) Provide arrows to show the mechanisms and then predict the products of the following acid base reaction. Use pKas to determine which way the reaction will favor (Hint: the lower pka acid will want to dissociate) Дон OH Ha OH NH2 c) H H-O-Harrow_forwardMATERIALS. Differentiate between interstitial position and reticular position.arrow_forwardFor each of the following, indicate whether the arrow pushes are valid. Do we break any rules via the arrows? If not, indicate what is incorrect. Hint: Draw the product of the arrow and see if you still have a valid structure. a. b. N OH C. H N + H d. e. f. مه N COHarrow_forward
- Decide which is the most acidic proton (H) in the following compounds. Which one can be removed most easily? a) Ha Нь b) Ha Нь c) CI CI Cl Ha Ньarrow_forwardProvide all of the possible resonanse structures for the following compounds. Indicate which is the major contributor when applicable. Show your arrow pushing. a) H+ O: b) c) : N :O : : 0 d) e) Оarrow_forwardDraw e arrows between the following resonance structures: a) b) : 0: :0: c) :0: N t : 0: بار Narrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you

 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning


Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning
Enzymes - Effect of cofactors on enzyme; Author: Tutorials Point (India) Ltd;https://www.youtube.com/watch?v=AkAbIwxyUs4;License: Standard YouTube License, CC-BY
Enzyme Catalysis Part-I; Author: NPTEL-NOC IITM;https://www.youtube.com/watch?v=aZE740JWZuQ;License: Standard Youtube License