
Concept explainers
a)
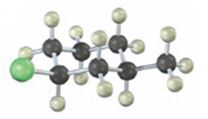
Interpretation:
The IUPAC name of the
Concept introduction:
The longest continuous carbon chain in the molecule is chosen. The chain is numbered from the end which gives lowest number to the substituent either halo or alkyl group present. If different halogens are present they are numbered and listed in the alphabetical order while writing the name. If same two alternatives exist for different substituents, then the chain is numbered from the end that gives lowest number to the substituent that has alphabetical preference.
To give:
The IUPAC name of the alkyl halide shown.
Answer to Problem 14VC
The alkyl halide given is
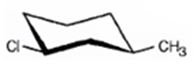
Its IUPAC name is cis- 1- chloro-3-methylcyclohexane.
Explanation of Solution
The compound has a cyclohexane ring with a Cl atom on C1 and a methyl on C3 both at equatorial positions. Hence its name is cis- 1- chloro-3-methylcyclohexane.
The IUPAC name of the alkyl halide shown is cis- 1- chloro-3-methylcyclohexane.
b)
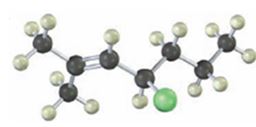
Interpretation:
The IUPAC name of the alkyl halide is to be given.
Concept introduction:
The longest continuous carbon chain in the molecule is chosen. The chain is numbered from the end which gives lowest number to the substituent either halo or alkyl group present. If different halogens are present they are numbered and listed in the alphabetical order while writing the name. If same two alternatives exist for different substituents, then the chain is numbered from the end that gives lowest number to the substituent that has alphabetical preference.
To give:
The IUPAC name of the alkyl halide shown.
Answer to Problem 14VC
The alkyl halide given is
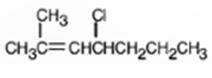
Its IUPAC name is 4-chloro-2-methyl-2-heptene.
Explanation of Solution
The compound has a seven carbon straight chain with a double bond between C2 & C3, a Cl atom on C4 and a methyl on C3. Hence its name is 4-chloro-2-methyl-2-heptene.
The IUPAC name of the alkyl halide shown is 4-chloro-2-methyl-2-heptene.
Want to see more full solutions like this?
Chapter 10 Solutions
Organic Chemistry
- Predict the intermediate 1 and final product 2 of this organic reaction: NaOMe ག1, ད།་, - + H You can draw 1 and 2 in any arrangement you like. 2 work up Note: if either 1 or 2 consists of a pair of enantiomers, just draw one structure using line bonds instead of 3D (dash and wedge) bonds at the chiral center. Explanation Check Click and drag to start drawing a structure. Х © 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Parrow_forwardWhat is the total energy cost associated with the compound below adopting the shown conformation? CH3 HH DH CH3arrow_forwardΗΝ, Draw Final Product C cyclohexanone pH 4-5 Edit Enamine H3O+ CH3CH2Br THF, reflux H Edit Iminium Ionarrow_forward
- How many hydrogen atoms are connected to the indicated carbon atom?arrow_forwardIdentify the compound with the longest carbon - nitrogen bond. O CH3CH2CH=NH O CH3CH2NH2 CH3CH2C=N CH3CH=NCH 3 The length of all the carbon-nitrogen bonds are the samearrow_forwardIdentify any polar covalent bonds in epichlorohydrin with S+ and 8- symbols in the appropriate locations. Choose the correct answer below. Η H's+ 6Η Η Η Η Η Ηδ Η Ο Ο HH +Η Η +Η Η Η -8+ CIarrow_forward
- H H:O::::H H H HH H::O:D:D:H HH HH H:O:D:D:H .. HH H:O:D:D:H H H Select the correct Lewis dot structure for the following compound: CH3CH2OHarrow_forwardRank the following compounds in order of decreasing boiling point. ннннн -С-С-Н . н-с- ННННН H ΗΤΗ НННН TTTĪ н-с-с-с-с-о-н НННН НН C' Н н-с-с-с-с-н НН || Ш НННН H-C-C-C-C-N-H ННННН IVarrow_forwardRank the following compounds in order of decreasing dipole moment. |>||>||| ||>|||>| |>|||>|| |||>||>| O ||>>||| H F H F H c=c || H c=c F F IIIarrow_forward
- choose the description that best describes the geometry for the following charged species ch3-arrow_forwardWhy isn't the ketone in this compound converted to an acetal or hemiacetal by the alcohol and acid?arrow_forwardWhat is the approximate bond angle around the nitrogen atom? HNH H Harrow_forward

 Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning

