
(a)
Interpretation:The mechanism for the formation of molecule 26 from molecule 28 through a hydride shift followed by deprotonation needs to be explained.
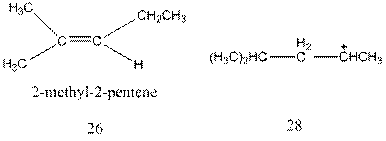
Concept Introduction : A carbocation rearrangement in which a hydrogen atom migrates from the carbon to the carbon possessing formal charge of one from the adjacent carbonis called hydride shift.
(b)
Interpretation:The mechanism for formation of 27 from 28 needs to be determined.
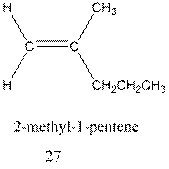
Concept Introduction : A carbocation rearrangement in which a hydrogen atom migrates from the carbon to the carbon possessing formal charge of one from the adjacent carbonis called hydride shift.
(c)
Interpretation:The mechanism of formation of 26 through acid catalysed rearrangement of either isomer of 4-methyl-2-pentene and formation of 27 by addition of a proton to 26 followed by deprotonation of intermediate carbocation needs to be explained.
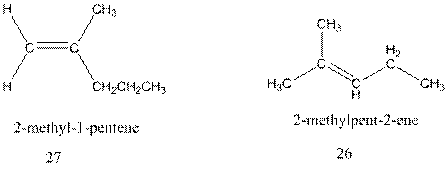
Concept Introduction : A carbocation rearrangement in which a hydrogen atom migrates from the carbon to the carbon possessing formal charge of one from the adjacent carbonis called hydride shift.
(d)
Interpretation:The different in the two types of mechanism for the formation of 26 and 27 needs to be explained.
Concept Introduction:Mechanism of a
Want to see the full answer?
Check out a sample textbook solution
Chapter 10 Solutions
EBK EXPERIMENTAL ORGANIC CHEMISTRY: A M
- Indicate the products obtained by mixing 2-Propanone and ethyllithium and performing a subsequent acid hydrolysis.arrow_forwardIndicate the products obtained if (E)-2-butenal and 3-oxo-butanenitrile are mixed with sodium ethoxide in ethanol.arrow_forwardQuestion 3 (4 points), Draw a full arrow-pushing mechanism for the following reaction Please draw all structures clearly. Note that this intramolecular cyclization is analogous to the mechanism for halohydrin formation. COH Br + HBr Brarrow_forward
- Indicate the products obtained if 2,2-dimethylpropanal and acetaldehyde are mixed with sodium ethoxide in ethanol.arrow_forwardIndicate the products obtained if 2,2-dimethylpropanal and acetaldehyde are reacted with sodium ethoxide in ethanol.arrow_forward2,2-Dimethylpropanal and acetaldehyde are reacted with sodium ethoxide in ethanol. Indicate the products obtained.arrow_forward
- Add conditions above and below the arrow that turn the reactant below into the product below in a single transformationADS fint anditions 百 Abl res condinese NC ง Add on condtions 1.0 B H,N.arrow_forward3. Provide all the steps and reagents for this synthesis. OHarrow_forwardSteps and explanationarrow_forward
- Steps and explanations please.arrow_forwardSteps on how to solve. Thank you!arrow_forward3. Name this ether correctly. H₁C H3C CH3 CH3 4. Show the best way to make the ether in #3 by a Williamson Ether Synthesis. Start from an alcohol or phenol. 5. Draw the structure of an example of a sulfide.arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





