
(a)
Interpretation:Whether norbornene is chiral or not should be explained.
Concept introduction:Chiral carbon is any stereocenter attached to four different alkyl substituents. If any two of the substituent happen to be similar the center is regarded as achiral. However, the most essential criteria that help to distinguish a chiral or non-chiral system ispresence of any symmetry element. If any plane center or axis of symmetry is identified it makes molecule achiral and optically inactive.
Four kinds of symmetry elements that may be present are tabulated as follows:
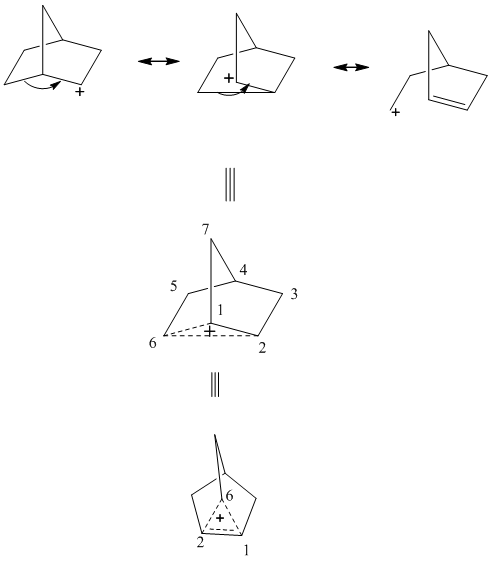
The non-classical cation is unique carbocation that involves delocalization of electron density of filled bonding orbital over three carbons. It exists for systems such as phenonium ions,norbornyltosylate, brosylate, and nortricyclonium cations. They can be understood as carbocation that has transition state for two asymmetric equilibrium cation derived from anchimeric-assistance that represents neighboring group participation in the stabilization of positive charge as illustrated below.
(b)
Interpretation:Three chiral centers in
Concept introduction:Chiral carbon is any stereocenter attached to four different alkyl substituents. If any two of the substituent happen to be similar the center is regarded as achiral. However, the most essential criteria that help to distinguish a chiral or non-chiral system ispresence of any symmetry element. If any plane center or axis of symmetry is identified it makes molecule achiral and optically inactive.
(c)
Interpretation: Chemical structure for the stereoisomer of
Concept introduction: Chiral carbon is any stereocenter attached to four different alkyl substituents. If any two of the substituent happen to be similar the center is regarded as achiral. However, the most essential criteria that help to distinguish a chiral or non-chiral system isthe presence of any symmetry element. If any plane center or axis of symmetry is identified it makes molecule achiral and optically inactive.
The enantiomers are identical chemical compounds that have a mirror-image relationship to each other while diastereomers do not hold mirror image relationships.
Want to see the full answer?
Check out a sample textbook solution
Chapter 10 Solutions
EBK EXPERIMENTAL ORGANIC CHEMISTRY: A M
- 3. Name this ether correctly. H₁C H3C CH3 CH3 4. Show the best way to make the ether in #3 by a Williamson Ether Synthesis. Start from an alcohol or phenol. 5. Draw the structure of an example of a sulfide.arrow_forward1. Which one(s) of these can be oxidized with CrO3 ? (could be more than one) a) triphenylmethanol b) 2-pentanol c) Ethyl alcohol d) CH3 2. Write in all the product(s) of this reaction. Label them as "major" or "minor". 2-methyl-2-hexanol H2SO4, heatarrow_forward3) Determine if the pairs are constitutional isomers, enantiomers, diastereomers, or mesocompounds. (4 points)arrow_forward
- In the decomposition reaction in solution B → C, only species C absorbs UV radiation, but neither B nor the solvent absorbs. If we call At the absorbance measured at any time, A0 the absorbance at the beginning of the reaction, and A∞ the absorbance at the end of the reaction, which of the expressions is valid? We assume that Beer's law is fulfilled.arrow_forward> You are trying to decide if there is a single reagent you can add that will make the following synthesis possible without any other major side products: 1. ☑ CI 2. H3O+ O Draw the missing reagent X you think will make this synthesis work in the drawing area below. If there is no reagent that will make your desired product in good yield or without complications, just check the box under the drawing area and leave it blank. Click and drag to start drawing a structure. Explanation Check ? DO 18 Ar B © 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center | Accessibilityarrow_forwardDon't use ai to answer I will report you answerarrow_forward
- Consider a solution of 0.00304 moles of 4-nitrobenzoic acid (pKa = 3.442) dissolved in 25 mL water and titrated with 0.0991 M NaOH. Calculate the pH at the equivalence pointarrow_forwardWhat is the name of the following compound? SiMe3arrow_forwardK Draw the starting structure that would lead to the major product shown under the provided conditions. Drawing 1. NaNH2 2. PhCH2Br 4 57°F Sunny Q Searcharrow_forward
- 7 Draw the starting alkyl bromide that would produce this alkyne under these conditions. F Drawing 1. NaNH2, A 2. H3O+ £ 4 Temps to rise Tomorrow Q Search H2arrow_forward7 Comment on the general features of the predicted (extremely simplified) ¹H- NMR spectrum of lycopene that is provided below. 00 6 57 PPM 3 2 1 0arrow_forwardIndicate the compound formula: dimethyl iodide (propyl) sulfonium.arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





