
Essential Organic Chemistry, Global Edition
3rd Edition
ISBN: 9781292089034
Author: Paula Yurkanis Bruice
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 10, Problem 70P
Phenolphthalein is an acid–base indicator. In solutions of pH < 8.5, it is colorless; in solutions of pH > 8.5, it is deep red-purple. Account for the change in color.
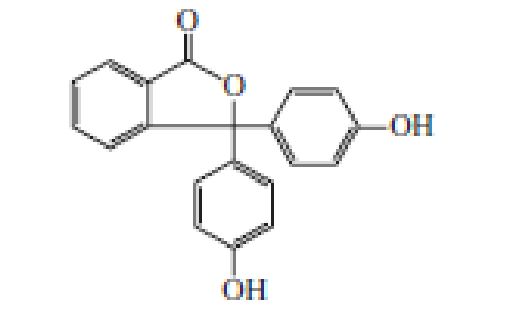
phenolphthalein
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
The normal pH of blood is 7.40 6 0.05 and is controlled in part by the H2CO3/HCO3- buffer system.(a) Assuming that the Ka value for carbonic acid at 25oC applies to blood, what is the [H2CO3]/[HCO3-] ratio in normal blood?(b) In a condition called acidosis, the blood is too acidic. What is the [H2CO3]/[HCO3-] ratio in a patient whose blood pH is 7.20?
Phenol red is a common acid-base indicator. It has a pKa equal to 8.000. Its
undissociated form is yellow and its anionic form is violet.
What is the concentration ratio between the acidic form vs. the basic form, [H-
PRED]/[PRed], of the pH = 7.50 solution?
Hint: Determine the concentration ratio of methyl red and its conjugate.
3. 40.00 mL of 0.0900 M NaOH is diluted to 100mL and titrated with 0.1000M HCI. Calculate the
pH after the addition of the following volumes of titrant (mL); (a) 0.00; (b) 10.00 ; (c) 18.00:
(d) 30.00 ; (e) 35.95 ; (f) 36.00 (g) 36.05 ; (h) 40.00.
Chapter 10 Solutions
Essential Organic Chemistry, Global Edition
Ch. 10.1 - Prob. 1PCh. 10.2 - What would distinguish the mass spectrum of...Ch. 10.2 - Prob. 3PCh. 10.3 - Prob. 5PCh. 10.3 - Suggest possible molecular formulas for a compound...Ch. 10.3 - Prob. 7PCh. 10.4 - Prob. 8PCh. 10.4 - Prob. 9PCh. 10.5 - Prob. 10PCh. 10.5 - Prob. 11P
Ch. 10.6 - Identify the ketone responsible for the mass...Ch. 10.6 - Prob. 13PCh. 10.8 - Prob. 14PCh. 10.8 - Prob. 15PCh. 10.12 - Which will occur at a larger wavenumber: a. a C :...Ch. 10.13 - Which will occur at a larger wavenumber: a. the C...Ch. 10.13 - Prob. 18PCh. 10.13 - Prob. 19PCh. 10.13 - Which will show an O 8 H stretch at a larger...Ch. 10.14 - Prob. 21PCh. 10.14 - Prob. 22PCh. 10.15 - Prob. 23PCh. 10.15 - Prob. 24PCh. 10.17 - Prob. 25PCh. 10.18 - Prob. 26PCh. 10.18 - Prob. 27PCh. 10.19 - Prob. 28PCh. 10.19 - Prob. 29PCh. 10.22 - How many signals would you expect to see in the 1H...Ch. 10.22 - Prob. 31PCh. 10.22 - Prob. 32PCh. 10.23 - Where would you expect to find the 1H NMR signal...Ch. 10.24 - Prob. 34PCh. 10.25 - Prob. 35PCh. 10.25 - Prob. 36PCh. 10.25 - Prob. 37PCh. 10.26 - Prob. 38PCh. 10.26 - Which of the following compounds is responsible...Ch. 10.27 - Prob. 40PCh. 10.27 - Prob. 41PCh. 10.27 - The 1H NMR spectra of two carboxylic acids with...Ch. 10.28 - Prob. 43PCh. 10.28 - Prob. 44PCh. 10.28 - Prob. 45PCh. 10.28 - Describe the 1H NMR spectrum you would expect for...Ch. 10.28 - Identify the compound with molecular formula...Ch. 10.29 - Prob. 48PCh. 10.29 - Prob. 49PCh. 10.29 - Identify the compound with a molecular formula of...Ch. 10 - In the mass spectrum of the following compounds,...Ch. 10 - For each of the following pairs of compounds,...Ch. 10 - Draw the structure of a saturated hydrocarbon that...Ch. 10 - Prob. 54PCh. 10 - Prob. 55PCh. 10 - How could you use UV spectroscopy to distinguish...Ch. 10 - Prob. 57PCh. 10 - Predict the relative intensities of the molecular...Ch. 10 - Prob. 59PCh. 10 - List the following compounds in order from highest...Ch. 10 - How can 1H NMR be used to prove that the addition...Ch. 10 - There are four esters with molecular formula...Ch. 10 - Prob. 63PCh. 10 - Prob. 64PCh. 10 - Each of the IR spectra presented here is...Ch. 10 - Prob. 66PCh. 10 - Five compounds are shown for each of the following...Ch. 10 - Prob. 68PCh. 10 - Prob. 69PCh. 10 - Phenolphthalein is an acidbase indicator. In...Ch. 10 - Which one of the following five compounds produced...Ch. 10 - Prob. 72PCh. 10 - Prob. 73PCh. 10 - Prob. 74PCh. 10 - How could 1H NMR distinguish between the compounds...Ch. 10 - Prob. 76PCh. 10 - Prob. 77PCh. 10 - The 1H NMR spectra of three isomers with molecular...Ch. 10 - The 1H NMR spectra of three isomers with molecular...Ch. 10 - Identify the following compounds. (Relative...Ch. 10 - An alkyl halide reacts with an alkoxide ion to...Ch. 10 - Determine the structure of a compound with...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Amino acids are an important group of compounds. At low pH, both the carboxylic acid group (CO2H) and the amine group (NHR) are protonated. However, as the pH of the solution increases (say, by adding base), the carboxylic acid proton is removed, usually at a pH between 2 and 3. In a middle range of pHs, therefore, the amine group is protonated, but the carboxylic acid group has lost the proton. (This is called a zwitterion.) At more basic pH values, the amine proton is dissociated. What is the pH of a 0.20 M solution of alanine hydrochloride, [NH3CHCH3CO2H]Cl?arrow_forwardThe acid-base indicator "Thymol Blue" has two transition ranges as listed below (with the corresponding acid dissociation constants): 7. Ka AcidColour BaseColour Thymol Blue 1 Thymol Blue 2 2.24 x 102 Red Yellow 1.26 x 10 Yellow Blue What colour would you expect it to be at the following pH values? Briefly justify your answers. (a) (b) (c) (d) 0.9 5.2 7.8 10.7arrow_forwardThe normal pH of blood is 7.40 ± 0.05 and is controlled in part by a H2CO3/HCO3 2 buffer system.(a) Assuming that the Ka value for carbonic acid at 25°C applies toblood, what is the [H2CO3]/[HCO3-] ratio in normal blood?(b) In a condition called acidosis, the blood is too acidic. What isthe [H2CO3]/[HCO3-] ratio in a patient whose blood pH is 7.20?arrow_forward
- An analytical chemist is titrating 211.2 mL of a 0.5700M solution of aniline (C6H5NH₂) with a 0.7200M solution of HNO3. The pK, of aniline is 9.37. Calculate the pH of the base solution after the chemist has added 140.1 mL of the HNO3 solution to it. Note for advanced students: you may assume the final volume equals the initial volume of the solution plus the volume of HNO3 solution added. Round your answer to 2 decimal places. pH = 0 P X Sarrow_forwardAn analytical chemist is titrating 57.3 mL of a 0.8800M solution of propylamine (C,H,NH,) with a 0.8400 M solution of HNO,. The p K; of propylamine is 3.46. Calculate the pH of the base solution after the chemist has added 69.1 mL of the HNO, solution to it. Note for advanced students: you may assume the final volume equals the initial volume of the solution plus the volume of HNO, solution added. Round your answer to 2 decimal places. pH =| doarrow_forwardA professor prepares a buffer solution that they need for the purification of protein from human cell lysates. They did mix weak acid and conjugate base and obtained the initial solution, which is characterised by these parameters. Final volume: 200mltotal buffer compound concentration: 150mMIntitial concentration weak acid: 0.03MInitial concentration conjugate base: 0.12MInitial pH 6.9buffer compound pka 6.3arrow_forward
- a of m-chlorobenzoic acid, HC7H4CIO2, is 1.5x 10-4. (a) Suppose buffer #1 is prepared using 40.0 mL 0.1 M HC7H4CIO2 and 60.0 mL 0.1 M C7H4CIO₂ to give a final volume of 100.0 mL. What is the pH of this buffer? 4.0 (b) Suppose a buffer #2 is prepared using 60.0 mL 0.1 M HC7H4ClO2 and 40.0 mL 0.1 M C7H4CIO₂ to give a final volume 100.0 mL. What is the pH of this buffer? 4.0 (c) Why is buffer #2 more acidic than buffer #1? O It has a greater volume of base. O It has a greater final volume. O It was prepared after buffer #1. It has a higher acid concentration. -arrow_forwardAn analytical chemest is titrating 150.6 ml. of a 0.6600 M solution of dhethylamine (C,H,) NH) wth a 0.1500M solution of HIO, The p K, of dethylamine is 2.89. Cakculate the pH of the base solution after the chemest has added 7773 ml. of the HIO, sohuton to . Note for advanced students: you may assume the fnal vokume equals the intal volume of the solution plas the volume of HIO, sokaton added. Round your answer to 2 decimal places please explain and solvelarrow_forwardAn analytical chemist is titrating 55.3 mL of a 0.8700M solution of aniline (CH,NH,) with a 0.2000M solution of HNO,. The p K, of aniline is 9.37. Calculate the pH of the base solution after the chemist has added 255.8 mL of the HNO, solution i to it. Note for advanced students: you may assume the final volume equals the initial volume of the solution plus the volume of HNO, solution added. Round your answer to 2 decimal places. pH = Explanation Check Acces Privacy O 2021 McGraw-Hill Education. All Rights Reserved. Terms of Use Warrow_forward
- Which of the following would be the best choice of a buffering system at pH 6.0? (a) H2SO4/HSO4-(b) Lactic Acid/Lactate(c) Carbonic Acid/Carbonate (d) Ammonium/ammoniaarrow_forwardAn analytical chemist is titrating 183.2 mL of a 0.1700M solution of aniline (C,H,NH,) with a 0.4700M solution of HNO,. The p K, of aniline is 9.37. Calculate the pH of the base solution after the chemist has added 10.6 mL of the HNO, solution to it. Note for advanced students: you may assume the final volume equals the initial volume of the solution plus the volume of HNO, solution added. Round your answer to 2 decimal places. pH = | ?arrow_forwardAn analytical chemist is titrating 139.8 mL of a 0.1600M solution of aniline (C,H,NH,) with a 0.6500M solution of HNO,. The p K, of aniline is 9.37. Calculate the pH of the base solution after the chemist has added 9.9 mL of the HNO, solution to it. Note for advanced students: you may assume the final volume equals the initial volume of the solution plus the volume of HNO, solution added. Round your answer to 2 decimal places. pH olo G) 18 Ar Explanation Check © 2022 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center | Accessibilityarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning
Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning


Principles of Modern Chemistry
Chemistry
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781133949640
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning
General Chemistry | Acids & Bases; Author: Ninja Nerd;https://www.youtube.com/watch?v=AOr_5tbgfQ0;License: Standard YouTube License, CC-BY