
Essential Organic Chemistry, Global Edition
3rd Edition
ISBN: 9781292089034
Author: Paula Yurkanis Bruice
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 10.28, Problem 46P
Describe the 1H NMR spectrum you would expect for each of the following compounds, Indicating the relative positions of the signals:
- a. BrCH2CH2CH2CH2Br
- b. CH3OCH2CH2CH2Br
- c. CH3CH2OCH2CH3
- d. CH3CH2OCH2Cl
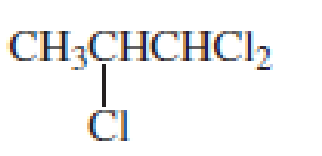
- e. CH3OCH2CH2CH2OCH3
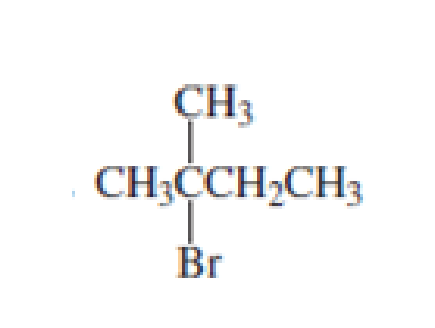
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
A. predict the number of 1H1H NMR signals for the compound (a)
B. Predict the number of 1H1H NMR signals for the compound (b).
C. Predict the number of 1H1H NMR signals for the compound (c).
D. Predict the number of 1H1H NMR signals for the compound (D).
A. Predict the splitting pattern of the compound (a) in the 1H1H NMR spectrum.
B. Predict the splitting pattern of the compound (b) in the 1H1H NMR spectrum.
C. Predict the splitting pattern of the compound (c) in the 1H1H NMR spectrum.
D. Predict the splitting pattern of the compound (d) in the 1H1H NMR spectrum.
Please don't provide handwriting solution
Chapter 10 Solutions
Essential Organic Chemistry, Global Edition
Ch. 10.1 - Prob. 1PCh. 10.2 - What would distinguish the mass spectrum of...Ch. 10.2 - Prob. 3PCh. 10.3 - Prob. 5PCh. 10.3 - Suggest possible molecular formulas for a compound...Ch. 10.3 - Prob. 7PCh. 10.4 - Prob. 8PCh. 10.4 - Prob. 9PCh. 10.5 - Prob. 10PCh. 10.5 - Prob. 11P
Ch. 10.6 - Identify the ketone responsible for the mass...Ch. 10.6 - Prob. 13PCh. 10.8 - Prob. 14PCh. 10.8 - Prob. 15PCh. 10.12 - Which will occur at a larger wavenumber: a. a C :...Ch. 10.13 - Which will occur at a larger wavenumber: a. the C...Ch. 10.13 - Prob. 18PCh. 10.13 - Prob. 19PCh. 10.13 - Which will show an O 8 H stretch at a larger...Ch. 10.14 - Prob. 21PCh. 10.14 - Prob. 22PCh. 10.15 - Prob. 23PCh. 10.15 - Prob. 24PCh. 10.17 - Prob. 25PCh. 10.18 - Prob. 26PCh. 10.18 - Prob. 27PCh. 10.19 - Prob. 28PCh. 10.19 - Prob. 29PCh. 10.22 - How many signals would you expect to see in the 1H...Ch. 10.22 - Prob. 31PCh. 10.22 - Prob. 32PCh. 10.23 - Where would you expect to find the 1H NMR signal...Ch. 10.24 - Prob. 34PCh. 10.25 - Prob. 35PCh. 10.25 - Prob. 36PCh. 10.25 - Prob. 37PCh. 10.26 - Prob. 38PCh. 10.26 - Which of the following compounds is responsible...Ch. 10.27 - Prob. 40PCh. 10.27 - Prob. 41PCh. 10.27 - The 1H NMR spectra of two carboxylic acids with...Ch. 10.28 - Prob. 43PCh. 10.28 - Prob. 44PCh. 10.28 - Prob. 45PCh. 10.28 - Describe the 1H NMR spectrum you would expect for...Ch. 10.28 - Identify the compound with molecular formula...Ch. 10.29 - Prob. 48PCh. 10.29 - Prob. 49PCh. 10.29 - Identify the compound with a molecular formula of...Ch. 10 - In the mass spectrum of the following compounds,...Ch. 10 - For each of the following pairs of compounds,...Ch. 10 - Draw the structure of a saturated hydrocarbon that...Ch. 10 - Prob. 54PCh. 10 - Prob. 55PCh. 10 - How could you use UV spectroscopy to distinguish...Ch. 10 - Prob. 57PCh. 10 - Predict the relative intensities of the molecular...Ch. 10 - Prob. 59PCh. 10 - List the following compounds in order from highest...Ch. 10 - How can 1H NMR be used to prove that the addition...Ch. 10 - There are four esters with molecular formula...Ch. 10 - Prob. 63PCh. 10 - Prob. 64PCh. 10 - Each of the IR spectra presented here is...Ch. 10 - Prob. 66PCh. 10 - Five compounds are shown for each of the following...Ch. 10 - Prob. 68PCh. 10 - Prob. 69PCh. 10 - Phenolphthalein is an acidbase indicator. In...Ch. 10 - Which one of the following five compounds produced...Ch. 10 - Prob. 72PCh. 10 - Prob. 73PCh. 10 - Prob. 74PCh. 10 - How could 1H NMR distinguish between the compounds...Ch. 10 - Prob. 76PCh. 10 - Prob. 77PCh. 10 - The 1H NMR spectra of three isomers with molecular...Ch. 10 - The 1H NMR spectra of three isomers with molecular...Ch. 10 - Identify the following compounds. (Relative...Ch. 10 - An alkyl halide reacts with an alkoxide ion to...Ch. 10 - Determine the structure of a compound with...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- AA compound with molecular formula C8H18 exhibits a 1H NMR spectrum with only one signal. How many signals would you expect in the 13C NMR spectrum of this compound? B.A compound with molecular formula C9H18 exhibits a 'H NMR spectrum with only one signal and a 13C NMR spectrum with two signals. Draw the structure of this compound. Draw Your Solution C. A compound with molecular formula C17H36 exhibits a ¹H NMR spectrum with only one signal. How many signals would you expect in the 13C NMR spectrum of this compound? D. Your answer is partially correct. For the following pair of compounds, identify how you would distinguish them using either ¹H NMR spectroscopy or 13C NMR spectroscopy. Enter the number of signals expected in each spectrum (enter as a number, e.g. "2"). Compound 1 Compound 2 Compound 1 is expected to have 2 signals in its ¹H NMR spectrum and 4 13C NMR spectrum. Compound 2 is expected to have 6 signals in its ¹H NMR spectrum and 10 signals in its 13C NMR spectrum. E.…arrow_forwardHow many 1H NMR signals does each natural product exhibit?arrow_forwardIdentify each of the following compounds from the 1H NMR data and molecular formula:a. C4H8Br2: a 6H singlet at 1.97 ppma 2H singlet at 3.89 ppm b. C8H9Br: a 3H doublet at 2.01 ppma 1H quartet at 5.14 ppma 5H broad singlet at 7.35 ppm c. C5H10O2: a 3H triplet at 1.15 ppma 3H triplet at 1.25 ppma 2H quartet at 2.33 ppma 2H quartet at 4.13 ppmarrow_forward
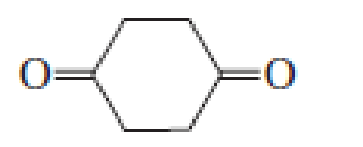
- Select the compound from each group that matches the HNMR spectrum shown below.arrow_forwardWhat is the number of signals that will be observed for each of the following molecules in the 1H NMR spectrum?arrow_forwardWhat compound(s) exhibits only two signals( a triplet & a quintet) in the 1H NMR spectrum? O BrCH2CH2CH2-O-CHCHCH₂Br BrCH2CH2CH2-O-CH2CH2CH2Br CICH2CH2CH2-O-CH2CH2CH2Br BrCH2CHCHCH₂Br BrCH2CH₂Br CH3CCCH3arrow_forward
- How many signals will the following compound display in the proton NMR spectrum? Compound: CH3CH2CH2COOCH2CH(CH3)2 O 6 O 7 O 3 O 5 4arrow_forwardHow many NMR signals would each compound show? -CH3CH2CH3 -CH3CH2OCH2CH3 -CH3CH2CH2OHarrow_forwardDibenzalacetone CDC13 11 10 7 6 4 3 2 1 ppm a. Match the hydrogens in dibenzalacetone to the signals in the spectrum. b. Does this sample also contain any benzaldehyde? c. Does this sample also contain any other impurities?arrow_forward
- a.) Annotate the 1H - NMR spectrum by labeling each signal (A-Z) and assigning them to the correct hydrogens on the structure of your unknown molecule Annotate the 13C- NMR spectrum by labeling each signal (A-Z) and then assign the A-Z labels to the correct carbons on the structure of your unknown molecule.arrow_forwardHow many unique 'H NMR and 1C NMR signals exist for each compound? CH3 А. C=C CH3 'H NMR signals in A 13C NMR signals in A H3C В. H3C CH3 'H NMR signals in B 13C NMR signals in Barrow_forward7. Identify the compound (CH₁0O) that gives the following ¹H NMR spectrum. SH 12 1H SH 8. Identify the compound (C8H₁1N) that gives the following ¹H NMR spectrum. Ho 2H PPM Ho 1H quartet PPM 2H 2H triplet triplet Fm Fo 3H doubletarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning Macroscale and Microscale Organic ExperimentsChemistryISBN:9781305577190Author:Kenneth L. Williamson, Katherine M. MastersPublisher:Brooks Cole
Macroscale and Microscale Organic ExperimentsChemistryISBN:9781305577190Author:Kenneth L. Williamson, Katherine M. MastersPublisher:Brooks Cole

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning

Macroscale and Microscale Organic Experiments
Chemistry
ISBN:9781305577190
Author:Kenneth L. Williamson, Katherine M. Masters
Publisher:Brooks Cole
NMR Spectroscopy; Author: Professor Dave Explains;https://www.youtube.com/watch?v=SBir5wUS3Bo;License: Standard YouTube License, CC-BY