
Concept explainers
In the mass spectrum of the following compounds, which would be more intense: the peak at m/z = 57 or the peak at m/z = 71?
- a. 3-methylpentane
- b. 2-methylpentane
Interpretation
The tallest peak should be identified.
Explanation of Solution
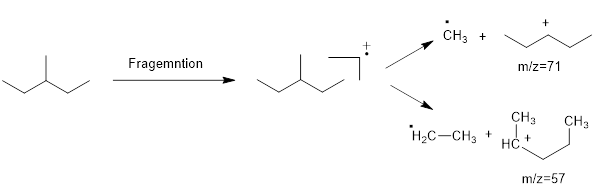
Mass fragmentation of 3-methyl pentane: In this molecule will be more adopted to lose an ethyl radical forming a secondary carbocation and a primary radical than a methyl radical forming secondary carbocation and methyl radical. In addition, 3-methylpentane ha undergoes for two path ways to lose an ethyl radical,
Therefore, the peak at
Mass fragmentation of 2-methyl pentane: The 2-methylpentane has undergoes for pathways to lose a methyl radical in each pathway and it cannot form a secondary carbocation by losing an ethyl radical. Loss of an ethyl radical would form a primary carbocation and a primary radical. Therefore, it will be more adopt to lose a methyl radical than an ethyl radical, the corresponding peak at
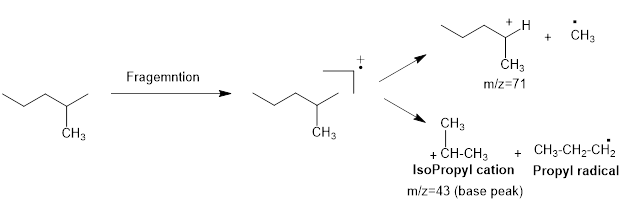
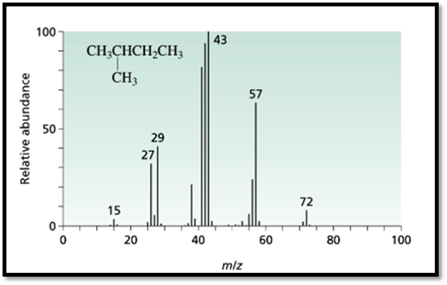
Figure 1
The 3-methyl pentane molecular peak at the m/e=71 has more intense then the peak at
Want to see more full solutions like this?
Chapter 10 Solutions
Essential Organic Chemistry, Global Edition
- Nucleophilic Aromatic Substitution: What is the product of the reaction? *see imagearrow_forwardShow the correct sequence to connect the reagent to product. * see imagearrow_forwardThe answer here says that F and K have a singlet and a doublet. The singlet and doublet are referring to the H's 1 carbon away from the carbon attached to the OH. Why don't the H's two carbons away, the ones on the cyclohexane ring, cause more peaks on the signal?arrow_forward
- Draw the Birch Reduction for this aromatic compound and include electron withdrawing groups and electron donating groups. *See attachedarrow_forwardShow the correct sequence to connect the reagent to product. * see imagearrow_forwardBlocking Group are use to put 2 large sterically repulsive group ortho. Show the correct sequence toconnect the reagent to product with the highest yield possible. * see imagearrow_forward
- Elimination-Addition: What molecule was determined to be an intermediate based on a “trapping experiment”? *please solve and see imagearrow_forwardShow the correct sequence to connect the reagent to product. * see imagearrow_forwardPredict the final product. If 2 products are made, list which should be “major” and “minor”. **see attachedarrow_forward

 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning

