
Essential Organic Chemistry, Global Edition
3rd Edition
ISBN: 9781292089034
Author: Paula Yurkanis Bruice
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 10, Problem 81P
An
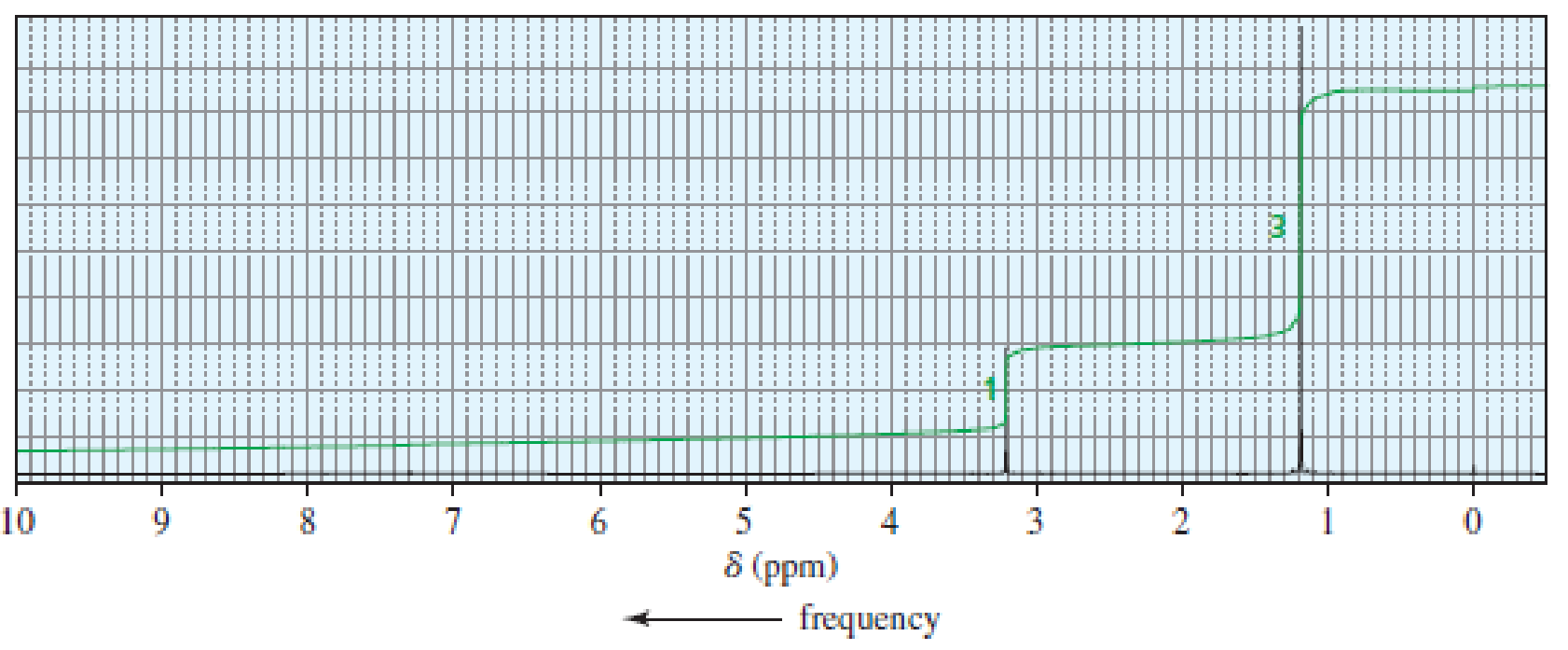
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
As we will learn in Chapter 20, reaction of (CH3)2CO with LiC ≡ CH followed by H2O affords compound D, which has a molecular ion in its mass spectrum at 84 and prominent absorptions in its IR spectrum at 3600–3200, 3303, 2938, and 2120 cm. D shows the following 1H NMR spectral data: 1.53 (singlet, 6 H), 2.37 (singlet, 1 H), and 2.43 (singlet, 1 H) ppm. What is the structure of D?
As we will learn in Chapter 17, reaction of (CH3)2CO with LIC≡CH followed by H2O affords compound D, which has a molecular ion in its mass spectrum at 84 and prominent absorptions in its IR spectrum at 3600−3200, 3303, 2938, and 2120 cm−1. D shows the following 1H NMR spectral data: 1.53 (singlet, 6 H), 2.37 (singlet, 1 H), and 2.43 (singlet, 1 H) ppm. What is the structure of D?
A compound is a ketone of molecular formula C7H14O. Its 13C NMR spectrum is shown in Figure. What is the structure of the compound?
Chapter 10 Solutions
Essential Organic Chemistry, Global Edition
Ch. 10.1 - Prob. 1PCh. 10.2 - What would distinguish the mass spectrum of...Ch. 10.2 - Prob. 3PCh. 10.3 - Prob. 5PCh. 10.3 - Suggest possible molecular formulas for a compound...Ch. 10.3 - Prob. 7PCh. 10.4 - Prob. 8PCh. 10.4 - Prob. 9PCh. 10.5 - Prob. 10PCh. 10.5 - Prob. 11P
Ch. 10.6 - Identify the ketone responsible for the mass...Ch. 10.6 - Prob. 13PCh. 10.8 - Prob. 14PCh. 10.8 - Prob. 15PCh. 10.12 - Which will occur at a larger wavenumber: a. a C :...Ch. 10.13 - Which will occur at a larger wavenumber: a. the C...Ch. 10.13 - Prob. 18PCh. 10.13 - Prob. 19PCh. 10.13 - Which will show an O 8 H stretch at a larger...Ch. 10.14 - Prob. 21PCh. 10.14 - Prob. 22PCh. 10.15 - Prob. 23PCh. 10.15 - Prob. 24PCh. 10.17 - Prob. 25PCh. 10.18 - Prob. 26PCh. 10.18 - Prob. 27PCh. 10.19 - Prob. 28PCh. 10.19 - Prob. 29PCh. 10.22 - How many signals would you expect to see in the 1H...Ch. 10.22 - Prob. 31PCh. 10.22 - Prob. 32PCh. 10.23 - Where would you expect to find the 1H NMR signal...Ch. 10.24 - Prob. 34PCh. 10.25 - Prob. 35PCh. 10.25 - Prob. 36PCh. 10.25 - Prob. 37PCh. 10.26 - Prob. 38PCh. 10.26 - Which of the following compounds is responsible...Ch. 10.27 - Prob. 40PCh. 10.27 - Prob. 41PCh. 10.27 - The 1H NMR spectra of two carboxylic acids with...Ch. 10.28 - Prob. 43PCh. 10.28 - Prob. 44PCh. 10.28 - Prob. 45PCh. 10.28 - Describe the 1H NMR spectrum you would expect for...Ch. 10.28 - Identify the compound with molecular formula...Ch. 10.29 - Prob. 48PCh. 10.29 - Prob. 49PCh. 10.29 - Identify the compound with a molecular formula of...Ch. 10 - In the mass spectrum of the following compounds,...Ch. 10 - For each of the following pairs of compounds,...Ch. 10 - Draw the structure of a saturated hydrocarbon that...Ch. 10 - Prob. 54PCh. 10 - Prob. 55PCh. 10 - How could you use UV spectroscopy to distinguish...Ch. 10 - Prob. 57PCh. 10 - Predict the relative intensities of the molecular...Ch. 10 - Prob. 59PCh. 10 - List the following compounds in order from highest...Ch. 10 - How can 1H NMR be used to prove that the addition...Ch. 10 - There are four esters with molecular formula...Ch. 10 - Prob. 63PCh. 10 - Prob. 64PCh. 10 - Each of the IR spectra presented here is...Ch. 10 - Prob. 66PCh. 10 - Five compounds are shown for each of the following...Ch. 10 - Prob. 68PCh. 10 - Prob. 69PCh. 10 - Phenolphthalein is an acidbase indicator. In...Ch. 10 - Which one of the following five compounds produced...Ch. 10 - Prob. 72PCh. 10 - Prob. 73PCh. 10 - Prob. 74PCh. 10 - How could 1H NMR distinguish between the compounds...Ch. 10 - Prob. 76PCh. 10 - Prob. 77PCh. 10 - The 1H NMR spectra of three isomers with molecular...Ch. 10 - The 1H NMR spectra of three isomers with molecular...Ch. 10 - Identify the following compounds. (Relative...Ch. 10 - An alkyl halide reacts with an alkoxide ion to...Ch. 10 - Determine the structure of a compound with...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- 2. The following synthesis was planned. The 'H NMR spectrum of the final product was run and is presented below. Was the synthesis successful? If not, what is the actual product and why? sos H2N' 10 로 1H 2H 2H 8 1. NaNO2 HCI (aq.) 2H 2H 6 2. H 1H 1H PPM 1H OH 1H HO 2arrow_forward9. Compound A is treated with HONO, followed by a reaction with Compound B; the final product obtained is shown below. H NMR spectra for Compounds A and B are provided. (a) Provide structures of Compounds A and B in the boxes below. (b) Label the signals on each spectrum with letters (as outlined on Week 3 Tuesday slide 4), then assign all H NMR signals to specific chemical environments or specific protons on the structures of Compounds A and B. Compound CompoundA HONO Fr Product H NMR Compound A 2H 1H 1H 1H 1H 3H H NMR Compound B 2H 1H 1H 2Harrow_forward2. The compound with the closed formula C4H9Br gives the following 1H NMR data. Write the clear formula of the compound with reasons. 1.0 ppm (triplet, 3 H); 1.7 ppm (dublet, 3H); 1.8 ppm (multiplet, 2H); 4.2 ppm (multiplet, 1H)arrow_forward
- 4. a. The ¹H NMR spectrum of a compound with the molecular formula C3H₁0O₂ is shown below (integral values are given above each set of peaks). Analysis of its ¹3C NMR spectrum shows peaks at 174, 61, 27, 14 and 9 ppm (see the posted lecture notes for June 7 for some guidance on ¹³C NMR spectroscopy). The IR spectrum shows a prominent peak at 1735 cm¹¹ and no peaks with a stretching frequency above 3000 cm³¹. What is the structure of this compound? 3.0 b. Which peak in the NMR spectrum in part a corresponds to a CH₂ group (called a "methylene") bonded to an O atom? a. 1.12 ppm b. 1.25 ppm c. 2.31 ppm e. There are no CH₂ groups bonded to an O atom in this compound. d. 4.12 ppm 1.0 PPMarrow_forward2. What is the structure of the compound which gives rise to this spectrum? Explain your reasoning. "H NMR spectrum doublet. 1.04 (6H multiplet. 6 1.95 (IH doublet. 8 3.35 (2H CH.CIarrow_forward5arrow_forward
- Using proton NMR spectra, how could a chemist distinguish between the following two compounds? H. H. СОСН Ph COCH; Ph H. Compound A Compound B The alkene protons in compound A display a larger coupling constant. Compound A and compound B show a different number of signals in the proton spectrum. The alpha alkene proton would be more shifted than the beta alkene proton in compound B Proton NMR spectroscopy cannot be used to distinguish between these compounds. The alkene protons in compound B display a larger coupling constant.arrow_forwardReaction between this aldehyde and ketone in base gives a compound A with the proton NMR spectrum: ô 1.10 (9H, s), 1.17 (9H, s), 6.4 (1H, d, J 15), and 7.0 (1H, d, J 15). What is its structure? (Don't forget stereochemistry!). When this compound reacts with HBr it gives compound B with this NMR spectrum: õu 1.08 (9H, s), 1.13 (9H, s), 2.71 (1H, dd, J 1.9, 17.7), 3.25 (dd, J10.0, 17.7), and 4.38 (1H, dd, J 1.9, 10.0). Suggest a structure, assign the spectrum, and give a mechanism for the formation of B. H base C11H₂00 HBr B C11H₂1 Broarrow_forwardPlease explain neatly and clearly!!!arrow_forward
- 13. Compound A has molecular formula C8H18. It shows one singlet in the 1H-NMR spectrum. Identify A and explain your reasoning.arrow_forward4. The 1H NMR spectrum shown below corresponds to one of the molecules A-D. Identify the molecule and then provide the complete IUPAC name for the molecule. Don't forget about stereochemistry. он ÕH ÕH B D 11 10 8 6 5 1 'H NMR: 3.35 (1H, m), 1.66 (1H, m), 1.36 (9H, m, overlapping signals), 0.91 (6H, d), 0.90 (3H, t)arrow_forwardThis compound, (C10H1002), shows strong, sharp absorption between 1700 and 1720 cm, and strong, broad absorption over the region 2500- 3000 cm. Given this information and the following NMR data, please draw the structure of the compound in the box below. H-NMR: 2.34 ppm, s(3H); 6.38 ppm, d(1H); 7.18 ppm, d(1H); 7.44 ppm, d(2H); 7.56 ppm, d(2H); 12.0, s(1H) 19C-NMR: 167.82; 143.82; 139.96; 131.45; 129.37; 127.83; 111.89; 21.13arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY
NMR Spectroscopy; Author: Professor Dave Explains;https://www.youtube.com/watch?v=SBir5wUS3Bo;License: Standard YouTube License, CC-BY