
Essential Organic Chemistry, Global Edition
3rd Edition
ISBN: 9781292089034
Author: Paula Yurkanis Bruice
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 10, Problem 79P
The 1H NMR spectra of three isomers with molecular formula C7H14O are shown here. Which isomer produces which spectrum?
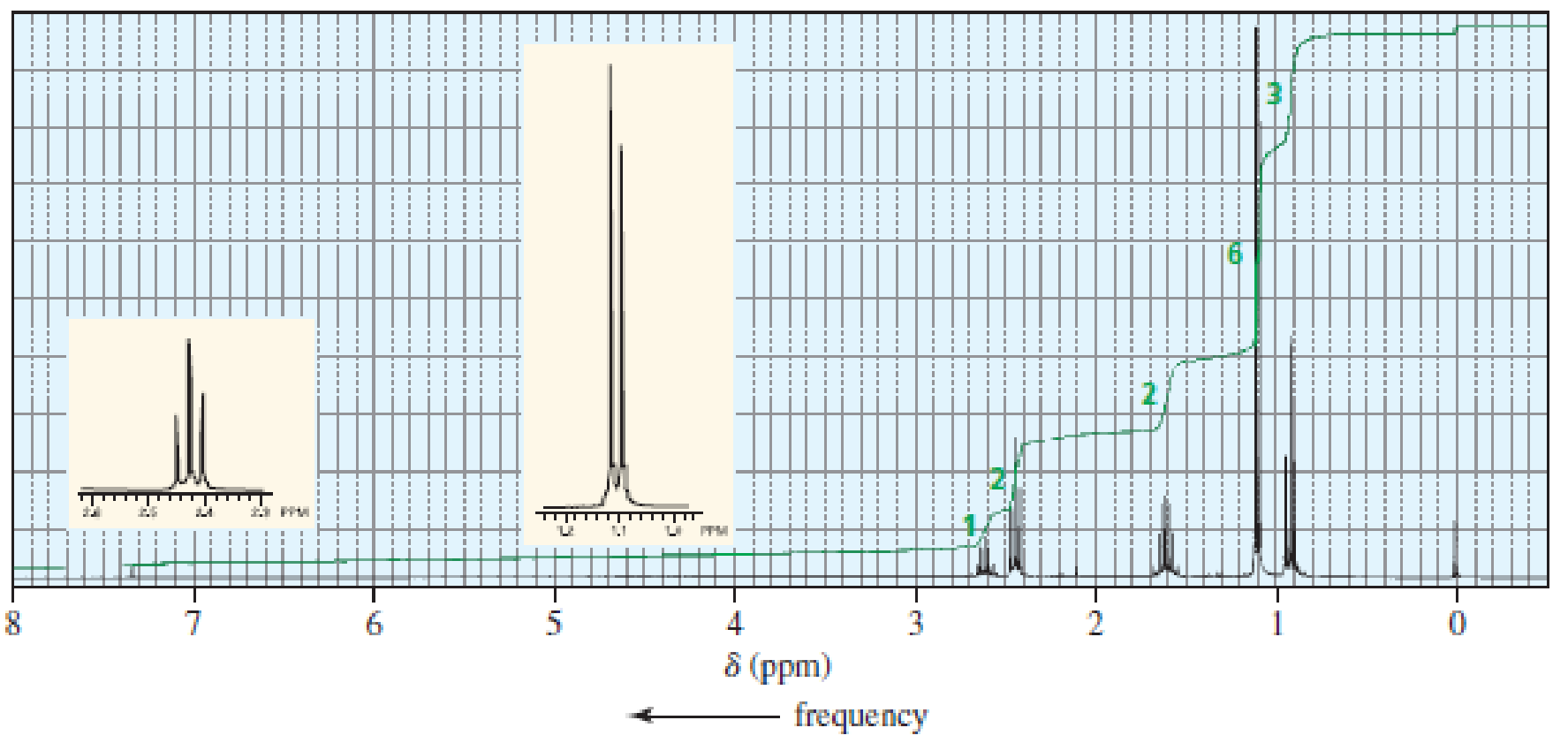
- a.
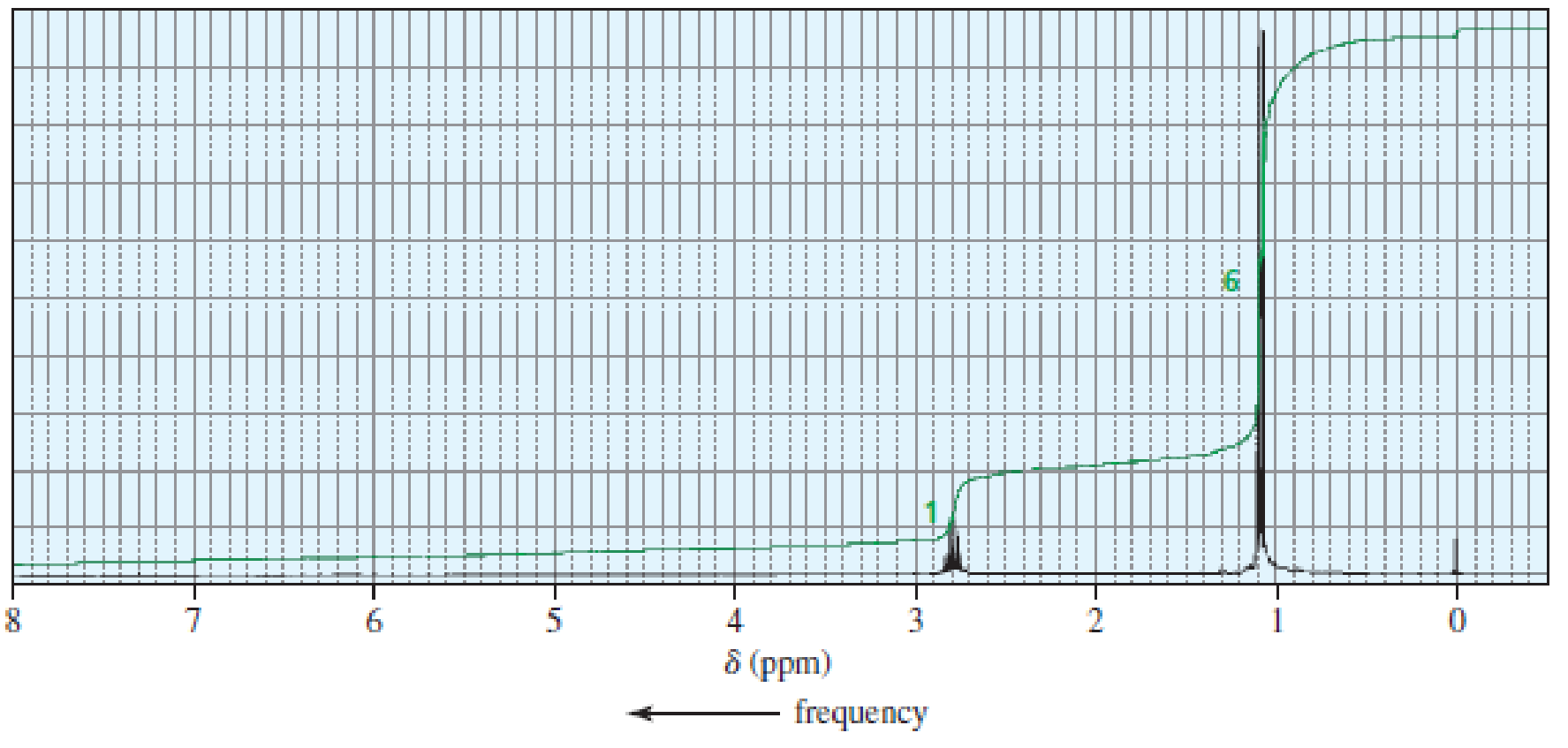
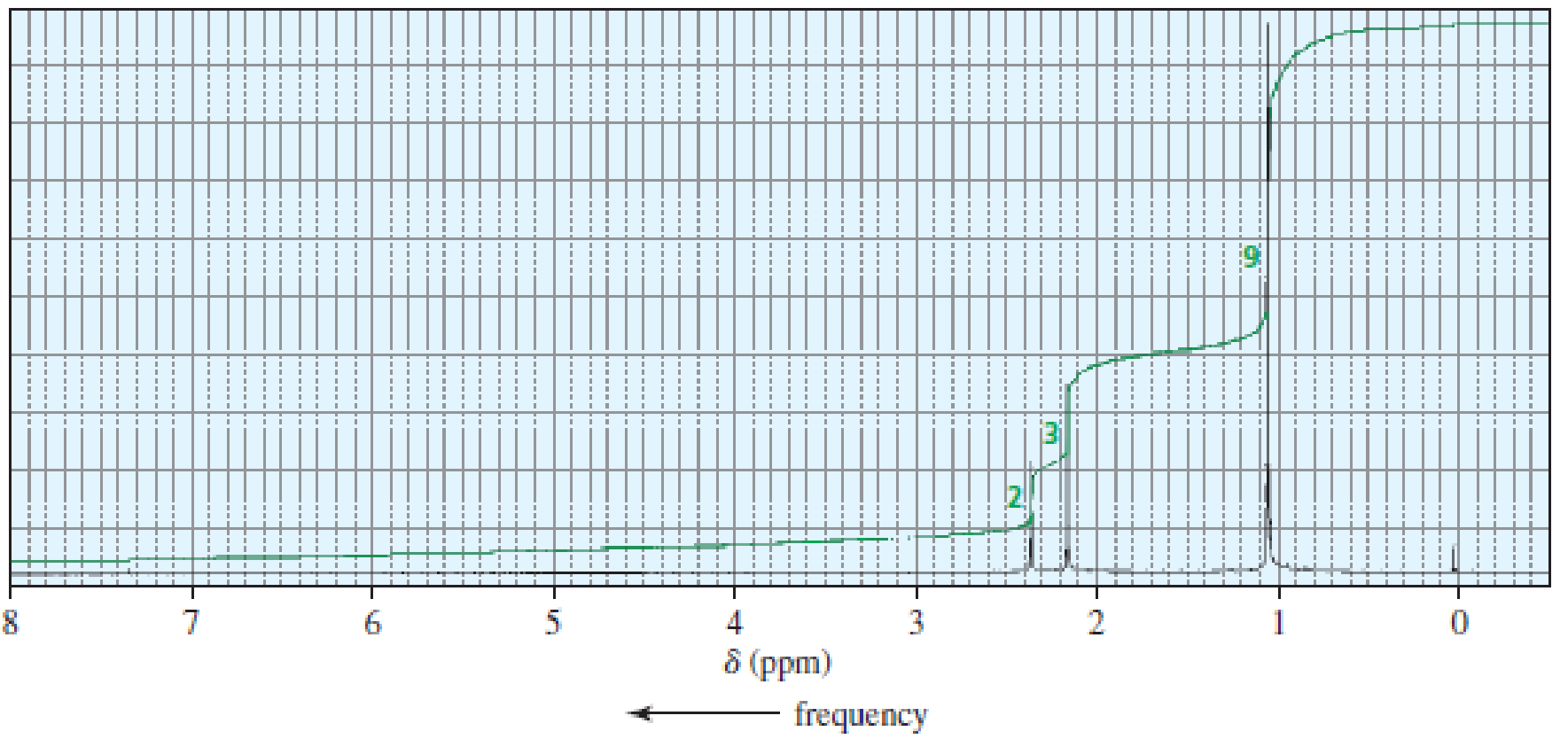
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
The 1H NMR spectra of three isomers with molecular formula C7H14O are shown here. Which isomer produces which spectrum?
A'2
The 'H-NMR spectra of cyclohexanol and cyclohexanone are given below.
Identify which spectrum belongs to which compound and assign the peaks in each spectrum that substantiate
your decision.
CH3
2. An unknown isomer of C4H9Cl has the ¹³C NMR and DEPT-135 spectra shown below:
13C NMR:
90
70
||||
A
50
8 (ppm)
CH3CH₂CH₂ CH₂ CI
30
10
DEPT-135:
CH3
CH3-CHCH₂-CI
B
90
70
Circle the compound that is represented by these spectra. Clearly explain your reasoning.
you will need to explain how each of the other three isomers was eliminated
from consideration.
50
8 (ppm)
CH3CH₂ CH-CI
CH3
C
30
10
CH3
CH3-C-CI
CH3
D
Chapter 10 Solutions
Essential Organic Chemistry, Global Edition
Ch. 10.1 - Prob. 1PCh. 10.2 - What would distinguish the mass spectrum of...Ch. 10.2 - Prob. 3PCh. 10.3 - Prob. 5PCh. 10.3 - Suggest possible molecular formulas for a compound...Ch. 10.3 - Prob. 7PCh. 10.4 - Prob. 8PCh. 10.4 - Prob. 9PCh. 10.5 - Prob. 10PCh. 10.5 - Prob. 11P
Ch. 10.6 - Identify the ketone responsible for the mass...Ch. 10.6 - Prob. 13PCh. 10.8 - Prob. 14PCh. 10.8 - Prob. 15PCh. 10.12 - Which will occur at a larger wavenumber: a. a C :...Ch. 10.13 - Which will occur at a larger wavenumber: a. the C...Ch. 10.13 - Prob. 18PCh. 10.13 - Prob. 19PCh. 10.13 - Which will show an O 8 H stretch at a larger...Ch. 10.14 - Prob. 21PCh. 10.14 - Prob. 22PCh. 10.15 - Prob. 23PCh. 10.15 - Prob. 24PCh. 10.17 - Prob. 25PCh. 10.18 - Prob. 26PCh. 10.18 - Prob. 27PCh. 10.19 - Prob. 28PCh. 10.19 - Prob. 29PCh. 10.22 - How many signals would you expect to see in the 1H...Ch. 10.22 - Prob. 31PCh. 10.22 - Prob. 32PCh. 10.23 - Where would you expect to find the 1H NMR signal...Ch. 10.24 - Prob. 34PCh. 10.25 - Prob. 35PCh. 10.25 - Prob. 36PCh. 10.25 - Prob. 37PCh. 10.26 - Prob. 38PCh. 10.26 - Which of the following compounds is responsible...Ch. 10.27 - Prob. 40PCh. 10.27 - Prob. 41PCh. 10.27 - The 1H NMR spectra of two carboxylic acids with...Ch. 10.28 - Prob. 43PCh. 10.28 - Prob. 44PCh. 10.28 - Prob. 45PCh. 10.28 - Describe the 1H NMR spectrum you would expect for...Ch. 10.28 - Identify the compound with molecular formula...Ch. 10.29 - Prob. 48PCh. 10.29 - Prob. 49PCh. 10.29 - Identify the compound with a molecular formula of...Ch. 10 - In the mass spectrum of the following compounds,...Ch. 10 - For each of the following pairs of compounds,...Ch. 10 - Draw the structure of a saturated hydrocarbon that...Ch. 10 - Prob. 54PCh. 10 - Prob. 55PCh. 10 - How could you use UV spectroscopy to distinguish...Ch. 10 - Prob. 57PCh. 10 - Predict the relative intensities of the molecular...Ch. 10 - Prob. 59PCh. 10 - List the following compounds in order from highest...Ch. 10 - How can 1H NMR be used to prove that the addition...Ch. 10 - There are four esters with molecular formula...Ch. 10 - Prob. 63PCh. 10 - Prob. 64PCh. 10 - Each of the IR spectra presented here is...Ch. 10 - Prob. 66PCh. 10 - Five compounds are shown for each of the following...Ch. 10 - Prob. 68PCh. 10 - Prob. 69PCh. 10 - Phenolphthalein is an acidbase indicator. In...Ch. 10 - Which one of the following five compounds produced...Ch. 10 - Prob. 72PCh. 10 - Prob. 73PCh. 10 - Prob. 74PCh. 10 - How could 1H NMR distinguish between the compounds...Ch. 10 - Prob. 76PCh. 10 - Prob. 77PCh. 10 - The 1H NMR spectra of three isomers with molecular...Ch. 10 - The 1H NMR spectra of three isomers with molecular...Ch. 10 - Identify the following compounds. (Relative...Ch. 10 - An alkyl halide reacts with an alkoxide ion to...Ch. 10 - Determine the structure of a compound with...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- The 1H and 13C NMR spectra of compound A, C8H9Br, are shown. Propose a structure for A, and assign peaks in the spectra to your structure.arrow_forwardPredict (draw) the ¹H NMR and 13C NMR spectra for each molecule. Your illustration should include chemical shift, integration, and splitting (where appropriate). Label each signal in your spectrum a, b, c... etc and label the corresponding C/H atoms in each molecule. CIarrow_forwardDraw all constitutional isomers of molecular formula C3H6Cl2.a. How many signals does each isomer exhibit in its 1H NMR spectrum?b. How many lines does each isomer exhibit in its 13C NMR spectrum?c. When only the number of signals in both 1H and 13C NMR spectroscopy is considered, is it possible to distinguish all of these constitutional isomers?arrow_forward
- Draw all constitutional isomers of molecular formula C3H6Cl2. a. How many signals does each isomer exhibit in its 1H NMR spectrum? b. How many lines does each isomer exhibit in its 13C NMR spectrum? c.When only the number of signals in both 1H and 13C NMR spectroscopy is considered, is it possible to distinguish all of these constitutional isomers?arrow_forwardThe 1H-NMR spectrum of ethanol shows a triplet at 1.23 ppm, a singlet at 2.61 pm, and a quartet at 3.69 ppm. Assign each signal to the protons it corresponds to in the molecule. Explain the splitting pattern observed for each signal.arrow_forwardHow many signals are present in the 1H NMR spectrum for each molecule? What splitting is observed in each signal?arrow_forward
- How many NMR signals/peaks does ethanol (CH3CH2OH) have? Identify the splitting pattern of each group/peak (is it a singlet, doublet, triplet, quartet, quintet, etc.).arrow_forwardCI (3d) Draw the DEPT-90 and DEPT-135 13C spectra for compound X. Label each carbon peak using your notation from (3a).arrow_forwardA3arrow_forward
- How many different 13C-absorption lines and how many 'H- resonances (disregard splitting and assume that solvent exchange of acidic hydrogens does NOT take place) are observed in the spectrum of each of the following compounds? (H3C)3C-C(CH3)2 CI A H3C (H3C)3C B CH3 CH3 H₂C= -COOH C CH-CI H3C-C-CH Cl Br Darrow_forwardOChem Help... Explain how you might distinguish between the following two compounds by comparing their 1H-NMR and their DEPT 135 spectra. Provide the bond line structures for the two compounds Use the correct splitting pattern AND approximate chemical shifts for the peaks given by each compound See attached image Also I did the bond line structures, but can you please check for correctnessarrow_forwardWhen the 1î-NMR spectrum of acetone, CH3COCH3, is recorded on an instrument operating at 200 MHz, a single sharp resonance at 2.1î is seen. (a) How many hertz downfield from TMS does the acetone resonance correspond to? (b) If the 1î-NMR spectrum of acetone were recorded at 500 MHz, what would the position of the absorption be in î units? (c) How many hertz downfield from TMS does this 500 MHz resonance correspond to?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you

IR Spectroscopy; Author: Professor Dave Explains;https://www.youtube.com/watch?v=_TmevMf-Zgs;License: Standard YouTube License, CC-BY