
Thinking Like an Engineer: An Active Learning Approach (4th Edition)
4th Edition
ISBN: 9780134639673
Author: Elizabeth A. Stephan, David R. Bowman, William J. Park, Benjamin L. Sill, Matthew W. Ohland
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 10, Problem 4RQ
- 4.
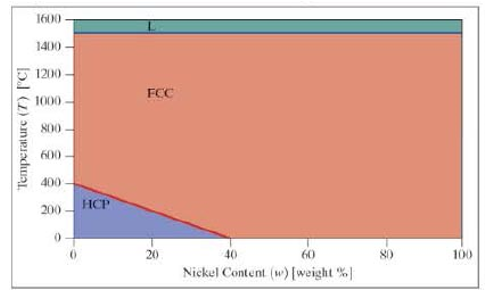 A simplified phase diagram for cobalt and nickel is shown. Assuming the lines shown are linear, we can say the mixture has the following characteristics:
A simplified phase diagram for cobalt and nickel is shown. Assuming the lines shown are linear, we can say the mixture has the following characteristics: - ■ Above 1500°C, it is a liquid.
- ■ Below 1500°C, there are two possible phases: face-centered cubic (FCC) phase and hexagonal close-packed (HCP) phase.
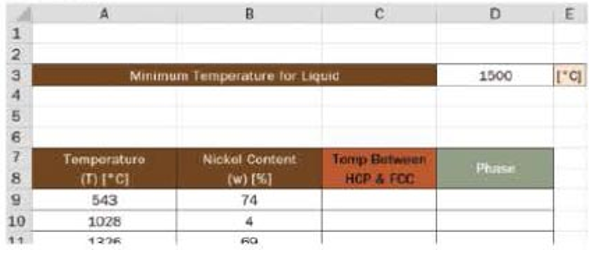 Use the workbook provided to determine the phase of a mixture, given the temperature and nickel content.
Use the workbook provided to determine the phase of a mixture, given the temperature and nickel content.
- a. Write the mathematical equation to describe the dividing line between the HCP region and the FCC region in column C. Reference the nickel content found in column B as needed. Add any absolute reference cells you feel are needed to complete this calculation.
- b. Write the conditional statement to determine the phase in column D. For simplicity, call the phases HCP, FCC, and L. For points on the line, YOU can decide which phase they are included in.
- c. Use conditional formatting to indicate each phase. Provide a color key.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
A wheel of diameter 150.0 mm and width 37.00 mm carrying a load 2.200 kN rolls on a
flat rail. Take the wheel material as steel and the rail material as cast iron. Assume the
figure given, which is based on a Poisson's ratio of 0.3, is applicable to estimate
the depth at which the maximum shear stress occurs for these materials. At this critical
depth, calculate the Hertzian stresses σr, σy, σz, and Tmax for the wheel.
1.0
0.8
0, т
Ratio of stress to Pmax
0.4
0.6
90
69
0.2
0.5b
b
1.5b
Tmax
2b
Distance from contact surface
The Hertizian stresses are as follows:
02 = or = -23.8 psi for the wheel =|
necessary.)
σy for the wheel =|
MPa
σz for the wheel =
MPa
V4 for the wheel = |
MPa
2.5b
ཡི
3b
MPa (Include a minus sign if
Only question 3
Only question 2
Chapter 10 Solutions
Thinking Like an Engineer: An Active Learning Approach (4th Edition)
Ch. 10.1 - Type 5 in cell E22 and 13 in cell E23; type =E22 +...Ch. 10.1 - Type 45 into cell G22 and =G22 + 10 in cell H22....Ch. 10.1 - Type 40 into cell A28 and =A28 + 10 in cell D28....Ch. 10.1 - Type 40 into cell A28 and =A28 + 5 in cell G28....Ch. 10.2 - Launch a new worksheet. Type the following Excel...Ch. 10.2 - As part of the design of a high-performance...Ch. 10.3 - Evaluate the following expressions. What is the...Ch. 10.3 - Prob. 8CCCh. 10.4 - This is a continuation of the worksheet you...Ch. 10.5 - Prob. 11CC
Ch. 10.6 - In 1980, the Environmental Protection Agency (EPA)...Ch. 10 - A B C D E F 1 2 45 3 meters 4...Ch. 10 - A B C D E F 1 2 45 3 meters 4...Ch. 10 - A B C D E F 1 2 45 3 meters 4...Ch. 10 - A B C D E F 1 2 45 3 meters 4...Ch. 10 - ICA 10-5 The worksheet shown here was designed to...Ch. 10 - The worksheet provided was designed to calculate...Ch. 10 - Some alternate energy technologies, such as wind...Ch. 10 - The worksheet shown was designed to calculate the...Ch. 10 - The worksheet shown was designed to calculate the...Ch. 10 - Refer to the following worksheet. The following...Ch. 10 - Write the output value that would appear in a cell...Ch. 10 - Write the output value that would appear in a cell...Ch. 10 - Refer to the following worksheet. In all...Ch. 10 - Prob. 14ICACh. 10 - A bioengineer conducts clinical trials on...Ch. 10 - Refer to the Worksheet shown, set up to calculate...Ch. 10 - You are interested in analyzing different implant...Ch. 10 - You have a large stock of several values of...Ch. 10 - We accidentally drop a tomato from the balcony of...Ch. 10 - You are interested in calculating the best place...Ch. 10 - 1. A history major of your acquaintance is...Ch. 10 - Prob. 2RQCh. 10 - 3. A phase diagram for carbon and platinum is...Ch. 10 - 4. A simplified phase diagram for cobalt and...Ch. 10 - 5. You enjoy drinking coffee but are particular...Ch. 10 - 6. In the 1950s, a team at Los Alamos National...Ch. 10 - Use the following phase diagram for questions 7...Ch. 10 - Use the following phase diagram for questions 7...Ch. 10 - Use the following phase diagram for questions 9...Ch. 10 - Use the following phase diagram for questions 9...Ch. 10 - 11. When liquid and vapor coexist in a container...Ch. 10 - 12. The ideal gas law assumes that molecules...Ch. 10 - One of the NAE Grand Challenges for Engineering is...Ch. 10 - 15 Create an Excel worksheet that will allow the...Ch. 10 - Prob. 16RQ
Additional Engineering Textbook Solutions
Find more solutions based on key concepts
Using your text editor, enter (that is, type in) the C++ program shown in Display 1.8. Be certain to type the f...
Problem Solving with C++ (10th Edition)
The solid steel shaft AC has a diameter of 25 mm and is supported by smooth bearings at D and E. It is coupled ...
Mechanics of Materials (10th Edition)
Write a summary list of the problem-solving steps identified in the chapter, using your own words.
BASIC BIOMECHANICS
How is the hydrodynamic entry length defined for flow in a pipe? Is the entry length longer in laminar or turbu...
Fluid Mechanics: Fundamentals and Applications
CONCEPT QUESTIONS
15.CQ3 The ball rolls without slipping on the fixed surface as shown. What is the direction ...
Vector Mechanics for Engineers: Statics and Dynamics
Why is the study of database technology important?
Database Concepts (8th Edition)
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, mechanical-engineering and related others by exploring similar questions and additional content below.Similar questions
- Solve for the support reactions at A and B. C 3 kN/m B -1.5 m- -1.5 m 1.5 m- 1.5 m-arrow_forward4. Solve for the support reactions at A and B. W1 600 lb/ft W2 150 lb/ft A Barrow_forwardIn cold isostatic pressing, the mold is most typically made of which one of the following: thermosetting polymer tool steel sheet metal textile rubberarrow_forward
- The coefficient of friction between the part and the tool in cold working tends to be: lower higher no different relative to its value in hot workingarrow_forwardThe force F={25i−45j+15k}F={25i−45j+15k} lblb acts at the end A of the pipe assembly shown in (Figure 1). Determine the magnitude of the component F1 which acts along the member AB. Determine the magnitude of the component F2 which acts perpendicular to the AB.arrow_forwardHi can you please help me with the attached question?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press
Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON
Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education
Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY
Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning
Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY
Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY

Elements Of Electromagnetics
Mechanical Engineering
ISBN:9780190698614
Author:Sadiku, Matthew N. O.
Publisher:Oxford University Press

Mechanics of Materials (10th Edition)
Mechanical Engineering
ISBN:9780134319650
Author:Russell C. Hibbeler
Publisher:PEARSON

Thermodynamics: An Engineering Approach
Mechanical Engineering
ISBN:9781259822674
Author:Yunus A. Cengel Dr., Michael A. Boles
Publisher:McGraw-Hill Education

Control Systems Engineering
Mechanical Engineering
ISBN:9781118170519
Author:Norman S. Nise
Publisher:WILEY

Mechanics of Materials (MindTap Course List)
Mechanical Engineering
ISBN:9781337093347
Author:Barry J. Goodno, James M. Gere
Publisher:Cengage Learning

Engineering Mechanics: Statics
Mechanical Engineering
ISBN:9781118807330
Author:James L. Meriam, L. G. Kraige, J. N. Bolton
Publisher:WILEY
Thermodynamics - Chapter 3 - Pure substances; Author: Engineering Deciphered;https://www.youtube.com/watch?v=bTMQtj13yu8;License: Standard YouTube License, CC-BY