
Thinking Like an Engineer: An Active Learning Approach (4th Edition)
4th Edition
ISBN: 9780134639673
Author: Elizabeth A. Stephan, David R. Bowman, William J. Park, Benjamin L. Sill, Matthew W. Ohland
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 10, Problem 3RQ
- 3. A phase diagram for carbon and platinum is shown. Assuming the lines shown are linear, we can say the mixture has the following characteristics:
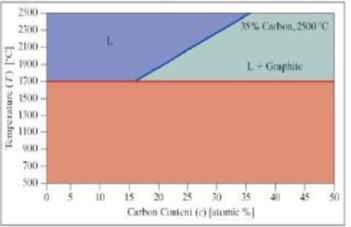
- Below 1700°C, it is a mixture of solid platinum and graphite.
- Above 1700°C, there are two possible phases: a liquid (L) phase and a liquid (L) + graphite phase. The endpoints of the division line between these two phases are labeled on the diagram.
Use the workbook provided to determine the phase of a mixture, given the temperature and carbon content.
| A | B | C | D | E | |
| 1 | |||||
| 2 | |||||
| 3 | Maximum Temperature for Pt + G | 1700 | [oC] | ||
| 4 | |||||
| 5 | |||||
| 6 | |||||
| 7 | Temperature (T) [oC] |
Carbon content (c) [%] |
Temp between L & L+G |
Phase | |
| 8 | |||||
| 9 | 854 | 42 | |||
| 10 | 564 | 20 | |||
- a. Write the equation to describe the temperature of the dividing line between the liquid (L) region and the liquid (L) 1 graphite region in column C. Reference the carbon content found in column B as needed. Add any absolute reference cells you feel are needed to complete this calculation.
- b. Write the conditional statement to determine the phase in column D. For simplicity, call the phases Pt + G, L, and L + G. For points on the line, YOU can decide which phase they are included in.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
got wrong answers help please
A crate weighs 530 lb and is hung by three ropes attached to
a steel ring at A such that the top surface is parallel to the
xy plane. Point A is located at a height of h = 42 in above
the top of the crate directly over the geometric center of the
top surface. Use the dimensions given in the table below to
determine the tension in each of the three ropes.
2013 Michael Swanbom
cc00
BY NC SA
↑ Z
C
b
B
У
a
D
Values for dimensions on the figure are given in the following
table. Note the figure may not be to scale.
Variable Value
a
30 in
b
43 in
4.5 in
The tension in rope AB is 383
x lb
The tension in rope AC is 156
x lb
The tension in rope AD is 156
x lb
A block of mass m hangs from the end of bar AB that is 7.2
meters long and connected to the wall in the xz plane. The
bar is supported at A by a ball joint such that it carries only a
compressive force along its axis. The bar is supported at end
B by cables BD and BC that connect to the xz plane at
points C and D respectively with coordinates given in the
figure. Cable BD is elastic and can be modeled as a linear
spring with a spring constant k = 400 N/m and unstretched
length of 6.34 meters.
Determine the mass m, the compressive force in beam AB
and the tension force in cable BC.
Z
C
D
(c, 0, d)
(a, 0, b)
A
B
y
f
m
cc 10
BY
NC SA
2016 Eric Davishahl
x
Values for dimensions on the figure are given in the following
table. Note the figure may not be to scale.
Variable Value
a
8.1 m
b
3.3 m
с
2.7 m
d
3.9 m
e
2 m
f
5.4 m
The mass of the block is 68.8
The compressive force in bar AB is
364
× kg.
× N.
The tension in cable BC is 393
× N.
Chapter 10 Solutions
Thinking Like an Engineer: An Active Learning Approach (4th Edition)
Ch. 10.1 - Type 5 in cell E22 and 13 in cell E23; type =E22 +...Ch. 10.1 - Type 45 into cell G22 and =G22 + 10 in cell H22....Ch. 10.1 - Type 40 into cell A28 and =A28 + 10 in cell D28....Ch. 10.1 - Type 40 into cell A28 and =A28 + 5 in cell G28....Ch. 10.2 - Launch a new worksheet. Type the following Excel...Ch. 10.2 - As part of the design of a high-performance...Ch. 10.3 - Evaluate the following expressions. What is the...Ch. 10.3 - Prob. 8CCCh. 10.4 - This is a continuation of the worksheet you...Ch. 10.5 - Prob. 11CC
Ch. 10.6 - In 1980, the Environmental Protection Agency (EPA)...Ch. 10 - A B C D E F 1 2 45 3 meters 4...Ch. 10 - A B C D E F 1 2 45 3 meters 4...Ch. 10 - A B C D E F 1 2 45 3 meters 4...Ch. 10 - A B C D E F 1 2 45 3 meters 4...Ch. 10 - ICA 10-5 The worksheet shown here was designed to...Ch. 10 - The worksheet provided was designed to calculate...Ch. 10 - Some alternate energy technologies, such as wind...Ch. 10 - The worksheet shown was designed to calculate the...Ch. 10 - The worksheet shown was designed to calculate the...Ch. 10 - Refer to the following worksheet. The following...Ch. 10 - Write the output value that would appear in a cell...Ch. 10 - Write the output value that would appear in a cell...Ch. 10 - Refer to the following worksheet. In all...Ch. 10 - Prob. 14ICACh. 10 - A bioengineer conducts clinical trials on...Ch. 10 - Refer to the Worksheet shown, set up to calculate...Ch. 10 - You are interested in analyzing different implant...Ch. 10 - You have a large stock of several values of...Ch. 10 - We accidentally drop a tomato from the balcony of...Ch. 10 - You are interested in calculating the best place...Ch. 10 - 1. A history major of your acquaintance is...Ch. 10 - Prob. 2RQCh. 10 - 3. A phase diagram for carbon and platinum is...Ch. 10 - 4. A simplified phase diagram for cobalt and...Ch. 10 - 5. You enjoy drinking coffee but are particular...Ch. 10 - 6. In the 1950s, a team at Los Alamos National...Ch. 10 - Use the following phase diagram for questions 7...Ch. 10 - Use the following phase diagram for questions 7...Ch. 10 - Use the following phase diagram for questions 9...Ch. 10 - Use the following phase diagram for questions 9...Ch. 10 - 11. When liquid and vapor coexist in a container...Ch. 10 - 12. The ideal gas law assumes that molecules...Ch. 10 - One of the NAE Grand Challenges for Engineering is...Ch. 10 - 15 Create an Excel worksheet that will allow the...Ch. 10 - Prob. 16RQ
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, mechanical-engineering and related others by exploring similar questions and additional content below.Similar questions
- The airplane weighs 144100 lbs and flies at constant speed and trajectory given by 0 on the figure. The plane experiences a drag force of 73620 lbs. 0 a.) If 11.3°, determine the thrust and lift forces = required to maintain this speed and trajectory. b.) Next consider the case where is unknown, but it is known that the lift force is equal to 7.8 times the quantity (Fthrust Fdrag). Compute the resulting trajectory angle and the lift force in this case. Use the same values for the weight and drag forces as you used for part a. 20. YAAY' Farag Ө Fthrust CC + BY NC SA 2013 Michael Swanbom Flift Fweight The lift force acts in the y' direction. The weight acts in the negative y direction. The thrust and drag forces act in the positive and negative x' directions respectively. Part (a) The thrust force is equal to 101,855 ☑ lbs. The lift force is equal to 141,282 ☑ lbs. Part (b) The trajectory angle 0 is equal to 7.31 ✓ deg. The lift force is equal to 143,005 ☑ lbs.arrow_forwardsimply supported beam has a concentrated moment M, applied at the left support and a concentrated force F applied at the free end of the overhang on the right. Using superposition, determine the deflection equations in regions AB and BC.arrow_forwardwhat is heat exchanger, what are formulas, and their importance, define the diagram, and give me a script on how to explain the design of heat exchanger, and how did values end up in that number. based on standards . what is dshellarrow_forward
- FIGURE P1.37 1.38 WP As shown in Figure P1.38, an inclined manometer is used to measure the pressure of the gas within the reservoir, (a) Using data on the figure, determine the gas pressure, in lbf/in.² (b) Express the pressure as a gage or a vacuum pressure, as appropriate, in lbf/in.² (c) What advantage does an inclined manometer have over the U-tube manometer shown in Figure 1.7? Patm = 14.7 lbf/in.² L I C i Gas a Oil (p = 54.2 lb/ft³) 140° 8=32.2 ft/s² 15 in.arrow_forwardwhat is an low pressure Heater, what are formulas, and their importance, define the diagram, and give me a script on how to explain the design of an air preheater, and how did values end up in that number. based on standardsarrow_forwardwhat is an air preheater, what are formulas, and their importance, define the diagram, and give me a script on how to explain the design of an air preheater, and how did values end up in that number. based on standardsarrow_forward
- Qf, Qa,Qm, Qcon,Qfg, Qbd, Qref,Qloss ( meaning, formula, percentage, and importance of higher value na qf, qa etc)arrow_forwardThe beam is supported by a fixed support at point C and a roller at point A. It also has an internal hinge at point B. The beam supports a point load at point D, a moment at point A and a distributed load on segment BC. a. calculate the support reactions at points A and C b. calculate the internal resultant loadings (N, V, M) at points E and F, which lies in the middle between points A and D P = 4 kip Ma = 5 kip-ft w1 = 3 kip/ft and w2 = 4 kip/ft a = 3 ftarrow_forwardFrom the image of the pyramid, I want to find what s1 hat, s2 hat, and s3 hat are. I think s3 hat is just equal to e3 hat right? What about the others?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press
Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON
Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education
Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY
Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning
Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY
Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY

Elements Of Electromagnetics
Mechanical Engineering
ISBN:9780190698614
Author:Sadiku, Matthew N. O.
Publisher:Oxford University Press

Mechanics of Materials (10th Edition)
Mechanical Engineering
ISBN:9780134319650
Author:Russell C. Hibbeler
Publisher:PEARSON

Thermodynamics: An Engineering Approach
Mechanical Engineering
ISBN:9781259822674
Author:Yunus A. Cengel Dr., Michael A. Boles
Publisher:McGraw-Hill Education

Control Systems Engineering
Mechanical Engineering
ISBN:9781118170519
Author:Norman S. Nise
Publisher:WILEY

Mechanics of Materials (MindTap Course List)
Mechanical Engineering
ISBN:9781337093347
Author:Barry J. Goodno, James M. Gere
Publisher:Cengage Learning

Engineering Mechanics: Statics
Mechanical Engineering
ISBN:9781118807330
Author:James L. Meriam, L. G. Kraige, J. N. Bolton
Publisher:WILEY
Mechanical Calculations; Author: Mometrix Academy;https://www.youtube.com/watch?v=FiQw8fpUHMY;License: Standard youtube license