
Introductory Chemistry: A Foundation
9th Edition
ISBN: 9781337399425
Author: Steven S. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 1, Problem 12ALQ
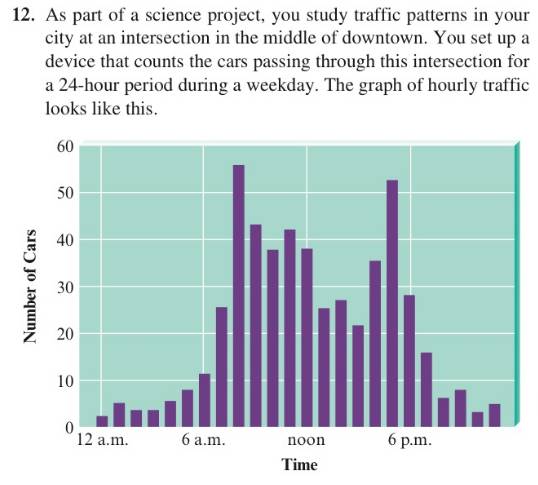
As part of a science project, you study traffic patterns in your city at an intersection in the middle of downtown. You set up a device that counts the cars passing through this intersection for a 24-hour period during a weekday. The graph of hourly traffic looks like this.
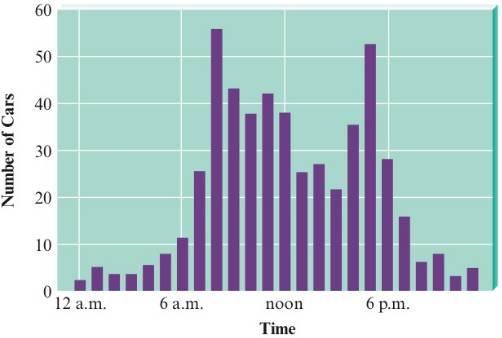
- At what lime(s) does the highest number of cars pass through the intersection?
- At what time(s) does the lowest number of cars pass through the intersection?
- Briefly describe the trend in numbers of cars over the course of the day.

Expert Solution & Answer
Trending nowThis is a popular solution!

Students have asked these similar questions
>
Can the molecule on the right-hand side of this organic reaction be made in good yield from no more than two reactants, in one
step, by moderately heating the reactants?
?
Δ
• If your answer is yes, then draw the reactant or reactants in the drawing area below. You can draw the reactants in any
arrangement you like.
If your answer is no, check the box under the drawing area instead.
Explanation
Check
Click and drag to start drawing a structure.
Х
© 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center | Acces
Predict the major products of the following organic reaction:
O O
+
A
?
Some important notes:
• Draw the major product, or products, of the reaction in the drawing area below.
• If there aren't any products, because no reaction will take place, check the box below the drawing area instead.
• Be sure to use wedge and dash bonds when necessary, for example to distinguish between major products that are
enantiomers.
Explanation Check
Click and drag to start drawing a structure.
eserved. Terms of Use | Privacy Center
>
(EXM 2, PRBLM 3) Here is this problem, can you explain it to me and show how its done. Thank you I need to see the work for like prbl solving.
Chapter 1 Solutions
Introductory Chemistry: A Foundation
Ch. 1.4 - What if everyone in the government used the...Ch. 1 - Discuss how a hypothesis can become a theory. Can...Ch. 1 - Make five qualitative and five quantitative...Ch. 1 - Prob. 3ALQCh. 1 - Differentiate between a “theory” and a “scientific...Ch. 1 - Describe three situations when you used the...Ch. 1 - Scientific models do not describe reality. They...Ch. 1 - Theories should inspire questions. Discuss a...Ch. 1 - Describe how you would set up an experiment to...Ch. 1 - If all scientists use the scientific method to try...
Ch. 1 - As stated in the text, there is no one scientific...Ch. 1 - In Section 1.3 the statement is made that it is...Ch. 1 - As part of a science project, you study traffic...Ch. 1 - Prob. 13ALQCh. 1 - Chemistry is an intimidating academic subject for...Ch. 1 - The first paragraphs in this chapter ask you if...Ch. 1 - This section presents several ways our day-to-day...Ch. 1 - The Chemistry in Focus segment titled Dr....Ch. 1 - This textbook provides a specific definition of...Ch. 1 - We use chemical reactions in our everyday lives,...Ch. 1 - Prob. 7QAPCh. 1 - Being a scientist is very much like being a...Ch. 1 - In science, what is the difference between a law...Ch. 1 - Observations may be either qualitative or...Ch. 1 - Prob. 11QAPCh. 1 - True or false? If a theory is disproven, then all...Ch. 1 - Although, in general, science has advanced our...Ch. 1 - Discuss several political, social, or personal...Ch. 1 - Although reviewing your lecture notes and reading...Ch. 1 - Why is the ability to solve problems important in...Ch. 1 - Students approaching the study of chemistry must...Ch. 1 - The ‘Chemistry in Focus” segmentChemistry: An...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- can someone draw out the reaction mechanism for this reaction showing all bonds, intermediates and side products Comment on the general features of the 1H-NMR spectrum of isoamyl ester provided belowarrow_forwardWhat would be the best choices for the missing reagents 1 and 3 in this synthesis? 1. PPh3 3 2. n-BuLi • Draw the missing reagents in the drawing area below. You can draw them in any arrangement you like. • Do not draw the missing reagent 2. If you draw 1 correctly, we'll know what it is. • Note: if one of your reagents needs to contain a halogen, use bromine. Click and drag to start drawing a structure.arrow_forwardIdentify the missing organic reactants in the following reaction: X + Y H+ two steps Note: This chemical equation only focuses on the important organic molecules in the reaction. Additional inorganic or small-molecule reactants or products (like H2O) are not shown. In the drawing area below, draw the skeletal ("line") structures of the missing organic reactants X and Y. You may draw the structures in any arrangement that you like, so long as they aren't touching. Click and drag to start drawing a structure. Х :arrow_forward
- I am struggling with the IUPAC (sys H Reply ☑Mark as Unreadarrow_forwardDon't used hand raiting and don't used Ai solution and correct answerarrow_forwardH R Part: 1/2 :CI: is a/an electrophile Part 2 of 2 Draw the skeletal structure of the product(s) for the Lewis acid-base reaction. Include lone pairs and formal charges (if applicable) on the structures. 4-7: H ö- H Skip Part Check X :C1: $ % L Fi Click and drag to start drawing a structure. MacBook Pro & ㅁ x G 0: P Add or increase positive formal cha Save For Later Submit ©2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Centearrow_forward
- Draw the friedel-crafts acylation mechanism of m-Xylenearrow_forwardDon't used hand raiting and don't used Ai solutionarrow_forward1. Base on this experimental results, how do you know that the product which you are turning in is methyl 3-nitrobenzoate(meta substituted product ) rather than either of the other two products? 2. What observation suggests that at least a small amount of one or both of the other two isomers are in the mother liquor?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
 Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning


Chemistry: The Molecular Science
Chemistry
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning
Types of Matter: Elements, Compounds and Mixtures; Author: Professor Dave Explains;https://www.youtube.com/watch?v=dggHWvFJ8Xs;License: Standard YouTube License, CC-BY