
Concept explainers
(a)
Interpretation:
The molecular formula for the given hydrocarbon has to be found.
Concept Introduction:
Organic compounds are the important basis of life. They include gasoline, coal, dyes, and clothing fibers etc. The compounds that are obtained from living organisms are termed as organic compounds and those obtained from the earth are known as inorganic compounds. Organic compounds are found in earth also apart from living organisms. All the organic compounds contain the element carbon. Urea was synthesized in the laboratory which is an organic compound.
Hydrocarbons are the organic compounds that contain only hydrogen and carbon atoms. Hydrocarbon derivatives are the one in which the compounds contain hydrogen and carbon atoms along with one or more additional elements. The additional elements that can be present in hydrocarbon derivatives are oxygen, nitrogen, sulphur, chlorine, bromine etc.
Hydrocarbons are further classified into two categories. They are saturated hydrocarbons and
Cycloalkanes are a class of saturated hydrocarbons that contain a ring of carbon atoms with or without alkyl substituents on it. The general molecular formula for cycloalkanes is
In a line-angle structural formula of cycloalkanes, the intersection of two lines represent a methylene group (
(a)
Answer to Problem 1.108EP
The molecular formula for the given hydrocarbon is
Explanation of Solution
Given name of cycloalkane is 1,2-dimethylcyclopentane. From the name it is understood that the parent cycloalkane is five carbon cyclic ring which is cyclopentane. The substituents present on the cyclopentane ring are two methyl groups, each at first and second carbon. Therefore, the line-angle structural formula for 1,2-dimethylcyclopentane can be drawn as shown below,
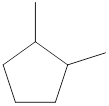
The above structure is a line-angle structural formula representation. This structure has a total of five intersections and two end lines. Therefore, the total number of carbon atoms present in the given structure is found to be seven.
The molecular formula for the given cycloalkane can be found using the general molecular formula. General molecular formula for cycloalkane is
The molecular formula for the given hydrocarbon is found to be
The molecular formula for the given hydrocarbon is found as
(b)
Interpretation:
The molecular formula for the given hydrocarbon has to be found.
Concept Introduction:
Organic compounds are the important basis of life. They include gasoline, coal, dyes, and clothing fibers etc. The compounds that are obtained from living organisms are termed as organic compounds and those obtained from the earth are known as inorganic compounds. Organic compounds are found in earth also apart from living organisms. All the organic compounds contain the element carbon. Urea was synthesized in the laboratory which is an organic compound.
Hydrocarbons are the organic compounds that contain only hydrogen and carbon atoms. Hydrocarbon derivatives are the one in which the compounds contain hydrogen and carbon atoms along with one or more additional elements. The additional elements that can be present in hydrocarbon derivatives are oxygen, nitrogen, sulphur, chlorine, bromine etc.
Hydrocarbons are further classified into two categories. They are saturated hydrocarbons and unsaturated hydrocarbons. The hydrocarbons that contain single bonds between carbon atoms in the entire molecule is known as saturated hydrocarbon. The hydrocarbons that contain atleast one double or triple bond between two carbon atoms in the entire molecule is known as unsaturated hydrocarbon.
Cycloalkanes are a class of saturated hydrocarbons that contain a ring of carbon atoms with or without alkyl substituents on it. The general molecular formula for cycloalkanes is
In a line-angle structural formula of cycloalkanes, the intersection of two lines represent a methylene group (
(b)
Answer to Problem 1.108EP
The molecular formula for the given hydrocarbon is
Explanation of Solution
Given name of cycloalkane is 1,3-dimethylcyclopentane. From the name it is understood that the parent cycloalkane is five carbon cyclic ring which is cyclopentane. The substituent present on the cyclopentane ring are two methyl groups, each at first and third carbon. Therefore, the line-angle structural formula for 1,3-dimethylcyclopentane can be drawn as shown below,

The above structure is a line-angle structural formula representation. This structure has a total of five intersections and two end lines. Therefore, the total number of carbon atoms present in the given structure is found to be seven.
The molecular formula for the given cycloalkane can be found using the general molecular formula. General molecular formula for cycloalkane is
The molecular formula for the given hydrocarbon is found to be
The molecular formula for the given hydrocarbon is found as
(c)
Interpretation:
Molecular formula for the given hydrocarbon has to be determined.
Concept Introduction:
Organic compounds are the important basis of life. They include gasoline, coal, dyes, and clothing fibers etc. The compounds that are obtained from living organisms are termed as organic compounds and those obtained from the earth are known as inorganic compounds. Organic compounds are found in earth also apart from living organisms. All the organic compounds contain the element carbon. Urea was synthesized in the laboratory which is an organic compound.
Hydrocarbons are the organic compounds that contain only hydrogen and carbon atoms. Hydrocarbon derivatives are the one in which the compounds contain hydrogen and carbon atoms along with one or more additional elements. The additional elements that can be present in hydrocarbon derivatives are oxygen, nitrogen, sulphur, chlorine, bromine etc.
Hydrocarbons are further classified into two categories. They are saturated hydrocarbons and unsaturated hydrocarbons. The hydrocarbons that contain single bonds between carbon atoms in the entire molecule is known as saturated hydrocarbon. The hydrocarbons that contain atleast one double or triple bond between two carbon atoms in the entire molecule is known as unsaturated hydrocarbon.
Molecular formula of the
(c)
Answer to Problem 1.108EP
The molecular formula for the given hydrocarbon is
Explanation of Solution
Given name of alkane is 2,2-dimethylpentane. From the name it is understood that the parent alkane is five carbon chain which is pentane. The substituent present on the hexane are two methyl groups, on the second carbon atom. Therefore, the line-angle structural formula for 2,2-dimethylpentane can be drawn as shown below,
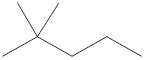
In line-angle structural representation of molecule, carbon atom is present where two lines meet and at the end of the lines. In the above structure there are three points where the lines meet and four end points. Therefore, the total number of carbon atoms present in the given structure is 7.
To determine the molecular formula of alkane, the value of “n” has to be substituted as 7 in the general formula of alkane.
Therefore, the molecular formula of the given hydrocarbon is determined as
The molecular formula of the hydrocarbon was determined as
(d)
Interpretation:
Molecular formula for the given hydrocarbon has to be determined.
Concept Introduction:
Organic compounds are the important basis of life. They include gasoline, coal, dyes, and clothing fibers etc. The compounds that are obtained from living organisms are termed as organic compounds and those obtained from the earth are known as inorganic compounds. Organic compounds are found in earth also apart from living organisms. All the organic compounds contain the element carbon. Urea was synthesized in the laboratory which is an organic compound.
Hydrocarbons are the organic compounds that contain only hydrogen and carbon atoms. Hydrocarbon derivatives are the one in which the compounds contain hydrogen and carbon atoms along with one or more additional elements. The additional elements that can be present in hydrocarbon derivatives are oxygen, nitrogen, sulphur, chlorine, bromine etc.
Hydrocarbons are further classified into two categories. They are saturated hydrocarbons and unsaturated hydrocarbons. The hydrocarbons that contain single bonds between carbon atoms in the entire molecule is known as saturated hydrocarbon. The hydrocarbons that contain atleast one double or triple bond between two carbon atoms in the entire molecule is known as unsaturated hydrocarbon.
Molecular formula of the alkanes can be found by using the general formula
(d)
Answer to Problem 1.108EP
The molecular formula for the given hydrocarbon is
Explanation of Solution
Given name of alkane is 2,3-dimethylpentane. From the name it is understood that the parent alkane is five carbon chain which is pentane. The substituent present on the hexane are two methyl groups, each at second and third carbon. Therefore, the line-angle structural formula for 2,3-dimethylpentane can be drawn as shown below,
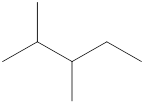
In line-angle structural representation of molecule, carbon atom is present where two lines meet and at the end of the lines. In the above structure there are three points where the lines meet and four end points. Therefore, the total number of carbon atoms present in the given structure is 7.
To determine the molecular formula of alkane, the value of “n” has to be substituted as 7 in the general formula of alkane.
Therefore, the molecular formula of the given hydrocarbon is determined as
The molecular formula of the given hydrocarbon was determined as
Want to see more full solutions like this?
Chapter 1 Solutions
Organic And Biological Chemistry
- Relative Abundance 20- Problems 501 (b) The infrared spectrum has a medium-intensity peak at about 1650 cm. There is also a C-H out-of-plane bending peak near 880 cm. 100- 80- 56 41 69 M(84) LL 15 20 25 30 35 55 60 65 70 75 80 85 90 m/zarrow_forwardPolyethylene furanoate is a polymer made from plant-based sources; it is used for packaging. Identify the monomer(s) used in the production of this polymer using a condensation process.arrow_forwardPhenol is the starting material for the synthesis of 2,3,4,5,6-pentachlorophenol, known al-ternatively as pentachlorophenol, or more simply as penta. At one time, penta was widely used as a wood preservative for decks, siding, and outdoor wood furniture. Draw the structural formula for pentachlorophenol and describe its synthesis from phenol.arrow_forward
- 12 Mass Spectrometry (d) This unknown contains oxygen, but it does not show any significant infrared absorption peaks above 3000 cm . 59 100- BO 40 Relative Abundance M(102) - 15 20 25 30 35 40 45 50 5 60 65 70 75 80 85 90 95 100 105 mizarrow_forwardDraw a Haworth projection of a common cyclic form of this monosaccharide: H HO H HO H HO H H -OH CH2OH Click and drag to start drawing a structure. Х : Darrow_forward: Draw the structure of valylasparagine, a dipeptide made from valine and asparagine, as it would appear at physiological pH. Click and drag to start drawing a structure. P Darrow_forward
- Draw the Haworth projection of α-L-mannose. You will find helpful information in the ALEKS Data resource. Click and drag to start drawing a structure. : ཊི Х Darrow_forwardDraw the structure of serine at pH 6.8. Click and drag to start drawing a structure. : d كarrow_forwardTake a look at this molecule, and then answer the questions in the table below it. CH2OH H H H OH OH OH CH2OH H H H H OH H H OH H OH Is this a reducing sugar? yes α β ロ→ロ no ☑ yes Does this molecule contain a glycosidic bond? If you said this molecule does contain a glycosidic bond, write the symbol describing it. O no 0+0 If you said this molecule does contain a glycosidic bond, write the common names (including anomer and enantiomer labels) of the molecules that would be released if that bond were hydrolyzed. If there's more than one molecule, separate each name with a comma. ☐arrow_forward
- Answer the questions in the table below about this molecule: H₂N-CH₂ -C—NH–CH–C—NH–CH—COO- CH3 CH CH3 What kind of molecule is this? 0= CH2 C If you said the molecule is a peptide, write a description of it using 3-letter codes separated ☐ by dashes. polysaccharide peptide amino acid phospolipid none of the above Хarrow_forwardDraw a Haworth projection of a common cyclic form of this monosaccharide: CH₂OH C=O HO H H -OH H OH CH₂OH Click and drag to start drawing a structure. : ☐ Х S '☐arrow_forwardNucleophilic Aromatic Substitution 22.30 Predict all possible products formed from the following nucleophilic substitution reactions. (a) (b) 9 1. NaOH 2. HCI, H₂O CI NH₁(!) +NaNH, -33°C 1. NaOH 2. HCl, H₂Oarrow_forward

 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co




