The Cy3 and Cy5 molecules were used in a FRET experiment. (e) Describe how the appearance of the donor and acceptor fluorescence spectrum will be different for the 12 base pair (bp) construct and the 21 base pair (bp) construct. Draw a spectrum for each.
5. The Cy3 and Cy5 molecules were used in a FRET experiment.
(e) Describe how the appearance of the donor and acceptor fluorescence spectrum will be different for the 12 base pair (bp) construct and the 21 base pair (bp) construct. Draw a spectrum for each.


FRET relies on the distance-dependent transfer of energy from a donor molecule to an acceptor molecule. Due to its sensitivity to distance, FRET has been used to investigate molecular interactions. A pair of molecules that interact in such a manner that FRET occurs is often referred to as a donor/acceptor pair. A type of FLUORESCENCE SPECTROSCOPY using two FLUORESCENT DYES with overlapping emission and absorption spectra, which is used to indicate the proximity of labeled molecules. This technique is useful for studying interactions of molecules and PROTEIN FOLDING.
Fluorescence Resonance Energy Transfer (FRET) is a physical phenomenon first described over 50 years ago, that is being used more and more in biomedical research and drug discovery today. FRET relies on the distance-dependent transfer of energy from a donor molecule to an acceptor molecule. Due to its sensitivity to distance, FRET has been used to investigate molecular interactions. FRET is the radiationless transmission of energy from a donor molecule to an acceptor molecule.
While there are many factors that influence FRET, the primary conditions that need to be met in order for FRET to occur are relatively few. The donor and acceptor molecules must be in close proximity to one another (typically 10-100 Å). The absorption or excitation spectrum of the acceptor must overlap the fluorescence emission spectrum of the donor. The degree to which they overlap is referred to as the spectral overlap integral (J). The donor and acceptor transition dipole orientations must be approximately parallel. Assuming that the donor-acceptor pairs are compatible the most critical element necessary for FRET to occur is close proximity of the pairs. Förster demonstrated that the efficiency of the process (E) depends on the inverse sixth-distance between donor and acceptor
E = Ro6/(Ro6 + r6)
Where Ro is the Förster distance at which half the energy is transferred and r is the actual distance between donor and acceptor. The distance at which energy transfer is 50% efficient is referred to as the Förster radius (Ro). The magnitude of the Ro is dependent on the spectral properties of the donor and the acceptor. Förster distances ranging from 20 to 90 Å are most useful for studies of biological macromolecules. These distances are comparable to the diameters of many proteins, the thickness of biological membranes, and the distances between sites on multisubunit proteins.
Ro=9.78 x 103(n-4*fd*k2*J)1/6 Å
As an example of the effect of distance on the efficiency of energy transfer, one can use some arbitrary numbers for the Förster distance (Ro) and the actual distance (r). If Ro is arbitrarily set to 1 (Ro =1), and the distance between the donor and acceptor is also equal to 1, then r=Ro and the equation for efficiency is E =16/(16 + 16), which is equal to 0.5 (i.e. 50%). This half-maximal value is what the Förster distance is defined as being. If the distance is 10 x closer (e.g. r=0.1Ro), then E = 16/(16 + 0.16) = 0.999999, considerably more efficient. However, if the distance between the donor and the acceptor is 10 x further away (i.e. r=10Ro), then E = 16/(16 + 106) = 0.0000001. This extreme sensitivity for distance is what allows FRET to be used for proximity experiments.
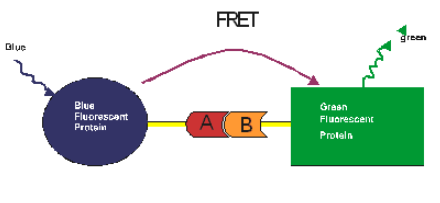
Genetically encoded fluorescent dyes, such as Green Fluorescent Protein (GFP) and related molecules blue, cyan, yellow and red have provided the ability to perform FRET in vitro, particularly in living cells [2]. These proteins form FRET pairs with each other as well as with conventional dyes. They can be attached to other proteins genetically or covalently yet still retain their fluorescent capability. These dyes have the utility of being genetic elements that can be linked with other genes to form chimeric proteins. These chimeric proteins contain a GFP (or related fluorescent protein element) and a putative binding domain. With different chimeric proteins (one donor and one acceptor) protein-protein interactions can be investigated. Only when the donor/acceptor pairs interacted through protein-protein interactions would FRET result.
The organic cyanine dyes Cy3, Cy5
Their emission range is such that background fluorescence is often reduced. Additionally, large distances (>100 Å) can be measured as a result of the high extinction coefficients and good quantum yields. Even donor-acceptor pairs with separated emission spectra (i.e. low overlap integral) result in acceptable Förster distances. For example, Cy3, which emits maximally at 570 nm, and Cy5, which emits at 670 nm, have a Förster distance >50 Å. Large separation between pairs allows the measurement of acceptor emission as a result of FRET without interference from donor emission.
Two complementary RNA oligonucleotides are labeled with Cy3 and Cy5 respectively. When these labeled molecules are not annealed (Figure 4A), excitation of an RNA oligonucleotide labeled with Cy3 with light at 540 nm results only in the emission of light by Cy3 at 590 nm, while the complementary RNA-oligo labeled with Cy5 does not emit any light at 590 nm or its true emission wavelength of 680 nm.
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images









