
Concept explainers
(a)
Interpretation:
The number of protons, neutrons and electrons present in the atom of
Concept Introduction:
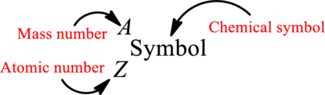
Mass number is the sum of the number of protons and neutrons inside the nucleus of an atom. This gives the number of subatomic particle present inside the nucleus. Mass number is represented by the symbol A.
From atomic number and mass number, the number of each sub atomic particle can be found.
Complete chemical symbol notation can be given as.
An element is a pure substance that cannot be broken by ordinary
(a)
Explanation of Solution
Given atom is
Atomic number is given as
Therefore, the number of protons is
(b)
Interpretation:
The number of protons, neutrons and electrons present in the atom of
Concept Introduction:
Refer part (a).
(b)
Explanation of Solution
Given atom is
Atomic number is given as
Therefore, the number of protons is
(c)
Interpretation:
The number of protons, neutrons and electrons present in the atom of tantalum-180 has to be given.
Concept Introduction:
Refer part (a).
(c)
Explanation of Solution
Given atom is tantalum-180. From this it is understood that the atomic number is
Atomic number is given as
Therefore, the number of protons is
(d)
Interpretation:
The number of protons, neutrons and electrons present in the atom of
Concept Introduction:
Refer part (a).
(d)
Explanation of Solution
Given atom is
Atomic number is given as
Therefore, the number of protons is
Want to see more full solutions like this?
Chapter F Solutions
CHEMICAL PRINCIPLES (LL) W/ACCESS
 Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
 General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning





