
Concept explainers
(a)
Interpretation:
The IUPAC name for the structure is to be given.
Concept introduction:
In the
Answer to Problem A.30P
The correct IUPAC name for the structure is
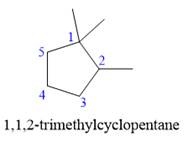
Explanation of Solution
The given structure is
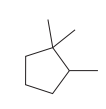
In the structure above, the ring has more carbon atoms than any of the straight chain groups. Hence, the root name of this structure is “cyclopentane.” The carbon atom of this ring, which is attached to two methyl groups, will be numbered as
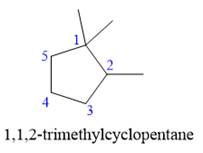
The correct IUPAC name for the given structure is
(b)
Interpretation:
The IUPAC name for the structure is to be given.
Concept introduction:
In the IUPAC nomenclature, the longest continuous carbon chain or the largest carbon ring is considered as the parent chain. The name of this corresponding parent chain/ring will be the root of the molecule’s name. Identify the substituents and add the name of the substituent as a prefix to the left of the root. The number assigned to the carbon that is bonded to the substituent is called the locator number or locant. In case of two or more different substituents, the alphabetical order is considered. Number each carbon atom of the chain sequentially, beginning with
Answer to Problem A.30P
The correct IUPAC name for the structure is
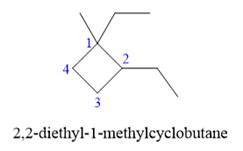
Explanation of Solution
The given structure is
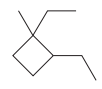
In the above structure, the ring has more carbon atoms than any of the straight chain groups. Hence, the root name of this structure is “cyclobutane.” The carbon atom of this ring, which is attached to two substituents, will be numbered as
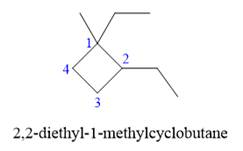
The correct IUPAC name for the given structure is
(c)
Interpretation:
The IUPAC name for the structure is to be given.
Concept introduction:
In the IUPAC nomenclature, the longest continuous carbon chain or the largest carbon ring is considered as the parent chain. The name of this corresponding parent chain/ring will be the root of the molecule’s name. Identify the substituents and add the name of the substituent as a prefix to the left of the root. The number assigned to the carbon that is bonded to the substituent is called the locator number or locant. In case of two or more different substituents, the alphabetical order is considered. Number each carbon atom of the chain sequentially, beginning with
Answer to Problem A.30P
The correct IUPAC name for the structure is
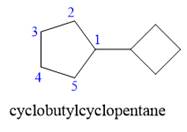
Explanation of Solution
The given structure is
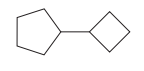
In the structure above, two rings are present. The ring on the left has more number of carbon atoms than the ring on the right; hence the root name of this compound is “cyclopentane.” The carbon atom of this cyclopentane ring to which the cyclobutane ring is attached will be numbered as

The correct IUPAC name for the given structure is
(d)
Interpretation:
The IUPAC name for the structure is to be given.
Concept introduction:
In the IUPAC nomenclature, the longest continuous carbon chain or the largest carbon ring is considered as the parent chain. The name of this corresponding parent chain/ring will be the root of the molecule’s name. Identify the substituents and add the name of the substituent as a prefix to the left of the root. The number assigned to the carbon that is bonded to the substituent is called the locator number or locant. In case of two or more different substituents, the alphabetical order is considered. Number each carbon atom of the chain sequentially, beginning with
Answer to Problem A.30P
The correct IUPAC name for the structure is
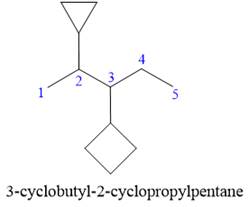
Explanation of Solution
The given structure is
In the structure above, there are two rings and a straight chain of carbon atoms. Out of these, the straight continuous carbon chain has more atoms than any of the rings. Hence, the straight chain carbon will serve as the parent, and the two rings will serve as substituents. Thus, the root name of this structure is “pentane.” The numbering of this parent chain should start from the left as the substituent encountered receives the lowest possible locator number. The two substituents are attached on the
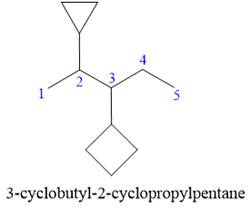
The correct IUPAC name for the given structure is
(e)
Interpretation:
The IUPAC name for the structure is to be given.
Concept introduction:
In the IUPAC nomenclature, the longest continuous carbon chain or the largest carbon ring is considered as the parent chain. The name of this corresponding parent chain/ring will be the root of the molecule’s name. Identify the substituents and add the name of the substituent as a prefix to the left of the root. The number assigned to the carbon that is bonded to the substituent is called the locator number or locant. In case of two or more different substituents, the alphabetical order is considered. Number each carbon atom of the chain sequentially, beginning with
Answer to Problem A.30P
The correct IUPAC name for the structure is
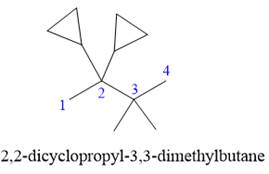
Explanation of Solution
The given structure is
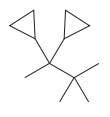
In the structure above, the straight continuous chain of carbon atoms will serve as the parent as it contains the highest number of carbon atoms than the rings. Hence, the root name of this structure is “butane.” There are two types of substituents attached- cyclopropyl and methyl. As the prefix “cyclo” is considered while naming, it will appear first in the IUPAC name. Thus, the numbering will also start from the left so that the carbon atom with two cyclopropyl rings will get the lowest possible locator numbers. The prefix di will be used for both the substituents as they appear twice. Thus, the IUPAC name for this structure is
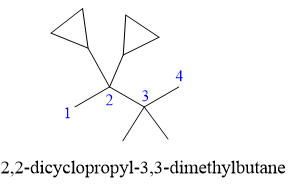
The correct IUPAC name for the given structure is
Want to see more full solutions like this?
Chapter A Solutions
Organic Chemistry: Principles and Mechanisms (Second Edition)
- 6. In an experiment the following replicate set of volume measurements (cm3) was recorded: (25.35, 25.80, 25.28, 25.50, 25.45, 25.43) A. Calculate the mean of the raw data. B. Using the rejection quotient (Q-test) reject any questionable results. C. Recalculate the mean and compare it with the value obtained in 2(a).arrow_forwardA student proposes the transformation below in one step of an organic synthesis. There may be one or more reactants missing from the left-hand side, but there are no products missing from the right-hand side. There may also be catalysts, small inorganic reagents, and other important reaction conditions missing from the arrow. • Is the student's transformation possible? If not, check the box under the drawing area. • If the student's transformation is possible, then complete the reaction by adding any missing reactants to the left-hand side, and adding required catalysts, inorganic reagents, or other important reaction conditions above and below the arrow. • You do not need to balance the reaction, but be sure every important organic reactant or product is shown. + T G OH де OH This transformation can't be done in one step.arrow_forwardMacmillan Leaming Draw the major organic product of the reaction. 1. CH3CH2MgBr 2. H+ - G Select Draw Templates More H о QQarrow_forward
- Draw the condensed structure of 3-hydroxy-2-butanone. Click anywhere to draw the first atom of your structure.arrow_forwardGive the expected major product of reaction of 2,2-dimethylcyclopropane with each of the following reagents. 2. Reaction with dilute H₂SO, in methanol. Select Draw Templates More CHC Erase QQQ c. Reaction with dilute aqueous HBr. Select Drew Templates More Era c QQQ b. Reaction with NaOCH, in methanol. Select Draw Templates More d. Reaction with concentrated HBr. Select Draw Templates More En a QQQ e. Reaction with CH, Mg1, then H*, H₂O 1. Reaction with CH,Li, then H', H₂Oarrow_forwardWrite the systematic name of each organic molecule: structure O OH OH name X ☐arrow_forward
- Macmillan Learning One of the molecules shown can be made using the Williamson ether synthesis. Identify the ether and draw the starting materials. А со C Strategy: Review the reagents, mechanism and steps of the Williamson ether synthesis. Determine which of the molecules can be made using the steps. Then analyze the two possible disconnection strategies and deduce the starting materials. Identify the superior route. Step 6: Put it all together. Complete the two-step synthesis by selecting the reagents and starting materials. C 1. 2. Answer Bank NaH NaOH NaOCH, снен, сен, он Сиси, Сне (СН), СОН (Сн, Свarrow_forwardWrite the systematic name of each organic molecule: structure CH3 O CH3-CH-CH-C-CH3 OH HV. CH3-C-CH-CH2-CH3 OH CH3 O HO—CH, CH–CH—C CH3 OH 오-오 name X G ☐arrow_forwardHI Organic Functional Groups Predicting the reactants or products of esterification What is the missing reactant in this organic reaction? HO OH H +回 + H₂O 60013 Naomi V Specifically, in the drawing area below draw the skeletal ("line") structure of R. If there is more than one reasonable answer, you can draw any one of them. If there is no reasonable answer, check the No answer box under the drawing area. No answer Click and drag to start drawing a structure. Explanation Check 1 2 #3 $ 4 2025 % ala5 'a :☐ G & 67 8 Ar K enter Accessible 9 Q W E R TY U 1 tab , S H J Karrow_forward
- Please help me with number 5 using my data and graph. I think I might have number 3 and 4 but if possible please check me. Thanks in advance!arrow_forwarddict the major products of this organic reaction. C Explanation Check 90 + 1.0₂ 3 2. (CH3)2S Click and drag f drawing a stru © 2025 McGraw Hill LLC. All Rights Reserved. • 22 4 5 7 8 Y W E R S F H Bilarrow_forwardcan someone draw out the reaction mechanism for this reaction showing all the curly arrows and 2. Draw the GPNA molecule and identify the phenylalanine portion. 3. Draw L-phenylalanine with the correct stereochemistryarrow_forward
