
Concept explainers
(a)
Interpretation:
The possible resonance structures for the following skeleton structure have to be determined. Also, the resonance structure that is the most important has to be determined.
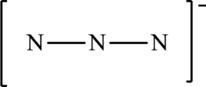
Concept Introduction:
The steps to draw the Lewis structure of the molecule are as follows:
Step 1: Find the central atom and place the other atoms around it. The atom in a compound that has the lowest group number or lowest electronegativity considered as the central atom.
Step 2: Estimate the total number of valence electrons.
Step 3: Connect the other atoms around the central atoms to the central atom with a single bond and lower the value of valence electrons by 2 of every single bond.
Step 4: Allocate the remaining electrons in pairs so that each atom can get 8 electrons.
The formula to calculate formal charge of the atom is as follows:
Some molecules and ions do not have one unique Lewis structure. The Lewis structures that differ only in the placement of multiple bonds are called resonance structures.
Resonance structures are defined as a set of two or more Lewis structures that collectively describe the electronic bonding. The actual bonding is an average of the bonding in the resonance structures. Also, not all resonance structures contribute equally in every case. Resonance structures that have high formal charges or that place charges of the same sign on adjacent atoms do not contribute to the bonding.
(a)
Answer to Problem 9.69QE
The possible resonance structures are,
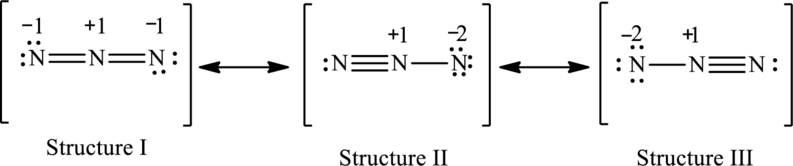
Structure I is more important.
Explanation of Solution
The given skeleton structure is,
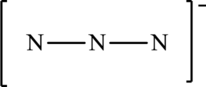
The resonance structures are as follows:
For structure I:
Substitute 5 for valence electrons, 4 for the number of lone pairs of electrons and 4 for the number of shared electrons in equation (1) to calculate the formal charge on first
Substitute 5 for valence electrons, 0 for number of lone pairs of electrons and 8 for the number of shared electrons in equation (1) to calculate the formal charge on second nitrogen atom.
Substitute 5 for valence electrons, 4 for number of lone pairs of electrons and 4 for the number of shared electrons in equation (1) to calculate the formal charge on third nitrogen atom.
For structure II:
Substitute 5 for valence electrons, 2 for the number of lone pairs of electrons and 6 for the number of shared electrons in equation (1) to calculate the formal charge on first
Substitute 5 for valence electrons, 0 for number of lone pairs of electrons and 8 for the number of shared electrons in equation (1) to calculate the formal charge on second nitrogen atom.
Substitute 5 for valence electrons, 6 for number of lone pairs of electrons and 2 for the number of shared electrons in equation (1) to calculate the formal charge on third nitrogen atom.
For structure III:
Substitute 5 for valence electrons, 6 for the number of lone pair of electrons and 2 for the number of shared electrons in equation (1) to calculate the formal charge on first
Substitute 5 for valence electrons, 0 for number of lone pairs of electrons and 8 for the number of shared electrons in equation (1) to calculate the formal charge on second nitrogen atom.
Substitute 5 for valence electrons, 2 for number of lone pairs of electrons and 6for the number of shared electrons in equation (1) to calculate the formal charge on third nitrogen atom.
The possible resonance structures with the formal charges are as follows:
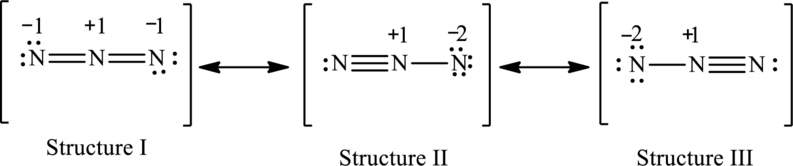
In these resonance structures, structure I have smallest formal charge because Lewis structures that have smallest formal charges are favored.
Hence, structure I is more important.
(b)
Interpretation:
The possible resonance structures for the following structure have to be determined. Also, the resonance structure that is the most important has to be determined.
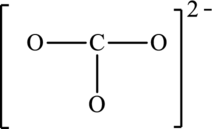
Concept introduction:
Refer to part (a)
(b)
Answer to Problem 9.69QE
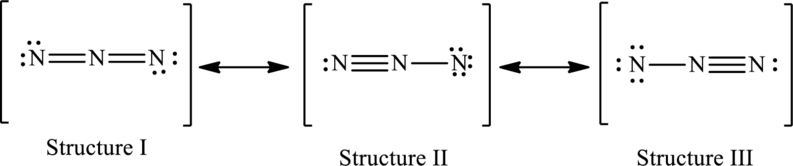
The possible resonance structures are as follows:
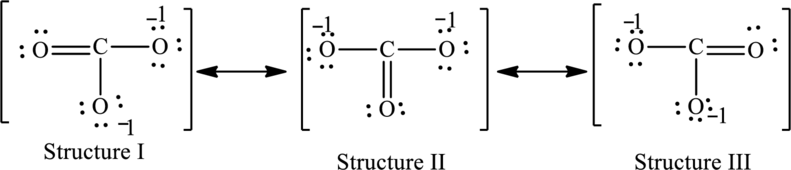
All structures are equally important.
Explanation of Solution
The given skeleton structure is,
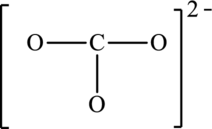
The resonance structures are as follows:
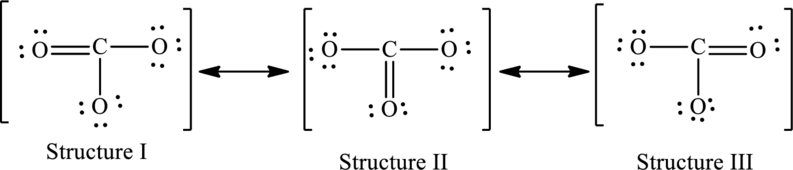
For structure I:
Substitute 4 for valence electrons, 0 for number of lone pairs of electrons and 8 for the number of shared electrons in equation (1) to calculate the formal charge on carbon atom.
Substitute 6 for valence electrons, 4 for number of lone pairs of electrons and 4for the number of shared electrons in equation (1) to calculate the formal charge on first oxygen atom.
Substitute 6 for valence electrons, 6 for number of lone pairs of electrons and 2 for the number of shared electrons in equation (1) to calculate the formal charge on second oxygen atom.
Substitute 6 for valence electrons, 6 for number of lone pairs of electrons and 2 for the number of shared electrons in equation (1) to calculate the formal charge on third oxygen atom.
For structure II:
Substitute 4 for valence electrons, 0 for number of lone pairs of electrons and 8 for the number of shared electrons in equation (1) to calculate the formal charge on carbon atom.
Substitute 6 for valence electrons, 6 for number of lone pairs of electrons and 2for the number of shared electrons in equation (1) to calculate the formal charge on first oxygen atom.
Substitute 6 for valence electrons, 4 for number of lone pairs of electrons and 4for the number of shared electrons in equation (1) to calculate the formal charge on second oxygen atom.
Substitute 6 for valence electrons, 6 for number of lone pairs of electrons and 2for the number of shared electrons in equation (1) to calculate the formal charge on third oxygen atom.
For structure III:
Substitute 4 for valence electrons, 0 for number of lone pairs of electrons and 8 for the number of shared electrons in equation (1) to calculate the formal charge on carbon atom.
Substitute 6 for valence electrons, 6 for number of lone pairs of electrons and 2 for the number of shared electrons in equation (1) to calculate the formal charge on first oxygen atom.
Substitute 6 for valence electrons, 6 for number of lone pairs of electrons and 2 for the number of shared electrons in equation (1) to calculate the formal charge on second oxygen atom.
Substitute 6 for valence electrons, 4 for number of lone pairs of electrons and 4 for the number of shared electrons in equation (1) to calculate the formal charge on third oxygen atom.
Possible resonance structures are as follows:
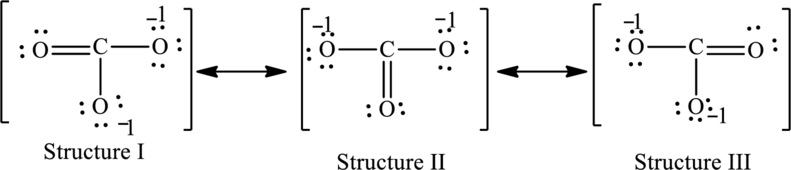
In these resonance structures, all the structures have the same formal charge. Hence, all the structures are equally important.
Want to see more full solutions like this?
Chapter 9 Solutions
Chemistry Principles And Practice
- Complete the following esterification reactions by drawing the line formulas of the carboxylic acid and alcohol required to form the ester shown. catalyst catalyst catalyst apricot fragrancearrow_forwardShow the saponification products of the following ester: You don't need to draw in the Na+ cation. catalyst, A catalyst, A catalyst, Aarrow_forwardWhat would happen if the carboxylic acid and alcohol groups were on the same molecule? In essence, the molecule reacts with itself. Draw the structure of the products formed in this manner using the reactants below. If two functional groups interact with one another on the same molecule, this is called an “intramolecular" (within one) rather than "intermolecular" (between two or more) attack. OH OH catalyst OH HO catalyst catalyst HO OHarrow_forward
- Q3: Write in the starting alkyl bromide used to form the following products. Include any reactants, reagents, and solvents over the reaction arrow. If more than one step is required, denote separate steps by using 1), 2), 3), etc. H OH racemic OH OH 5 racemicarrow_forwardDraw the Lewis structure of the SO3-O(CH3)2 complex shown in the bottom right of slide 2in lecture 3-3 (“Me” means a CH3 group) – include all valence electron pairs and formal charges.From this structure, should the complex be a stable molecule? Explain.arrow_forwardPredict all organic product(s), including stereoisomers when applicable.arrow_forward
- What I Have Learned Directions: Given the following reaction and the stress applied in each reaction, answer the question below. A. H2(g) + Cl2(g) 2 HCl(g) Stress applied: Decreasing the pressure 1. What is the Keq expression? 2. What will be the effect in the number of moles of HCl(g)? 3. What will be the Equilibrium Shift or the reaction? B. Fe3O4(s) + 4 H2(g) + heat 53 Fe(s) + 4 H₂O(g) Stress applied: Increasing the temperature 1. What is the Keq expression?. 2. What will be the effect in the volume of water vapor collected? 3. What will be the Equilibrium Shift or the reaction? C. 4 NH3(g) + 5 O2(g) 4 NO(g) + 6 H2O(g) + heat Stress applied: Increasing the volume of the container 1. What is the Keq expression?. 2. What will be the effect in the amount of H₂O? 3. What will be the Equilibrium Shift or the reaction?arrow_forwardConsider the solubility products (Ksp values) for the following compounds:SrSO4 (Ksp = 7.6 x 10−7), BaSO4 (Ksp = 1.5 x 10−9), SrCO3 (Ksp = 7.0 x 10−10), BaCO3 (Ksp = 1.6 x 10−9)Which anion is the harder base, CO32− or SO42−? Justify your answer.arrow_forwardQ1: a) Arrange the compounds in order of decreasing pKa, highest first. ОН ΟΗ ῸΗ дон ОН ОН CI Brarrow_forward
 Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning





