
Concept explainers
(a)
Interpretation:
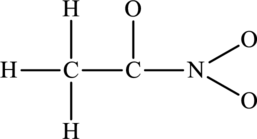
The possible resonance structures for the following skeleton structure have to be determined. Also, the most important resonance structure has to be identified.
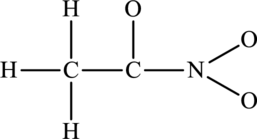
Concept Introduction:
The steps to draw the Lewis structure of the molecule are as follows:
Step 1: Find the central atom and place the other atoms around it. The atom in a compound that has the lowest group number or lowest electronegativity considered as the central atom.
Step 2: Estimate the total number of valence electrons.
Step 3: Connect the other atoms around the central atoms to the central atom with a single bond and lower the value of valence electrons by 2 of every single bond.
Step 4: Allocate the remaining electrons in pairs so that each atom can get 8 electrons.
The formula to calculate formal charge of the atom is as follows:
Some molecules and ions do not have one unique Lewis structure. The Lewis structures that differ only in the placement of multiple bonds are called resonance structures.
Resonance structures are defined as a set of two or more Lewis structures that collectively describe the electronic bonding. The actual bonding is an average of the bonding in the resonance structures. Also, not all resonance structures contribute equally in every case. Resonance structures that have high formal charges or that place charges of the same sign on adjacent atoms do not contribute to the bonding.
(a)
Answer to Problem 9.68QE
Possible resonance structures are as follows:
All resonance structures are equally important.
Explanation of Solution
The skeleton structure is as follows:
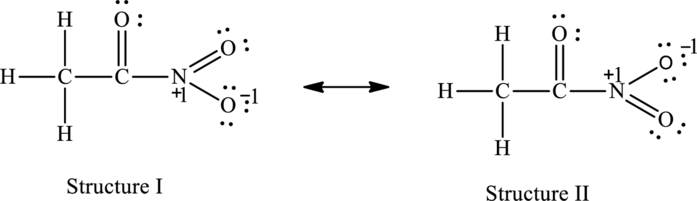
The resonance structures are as follows:
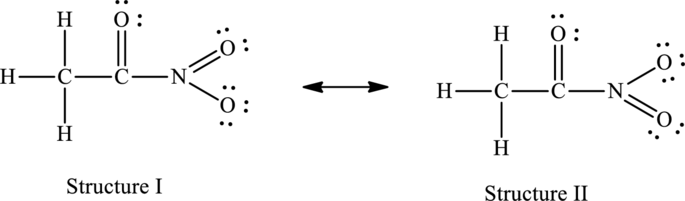
For structure I:
Substitute 5 for valence electrons, 0 for the number of lone pair of electrons and 8for the number of shared electrons in equation (1) to calculate the formal charge on nitrogen atom.
Substitute 6 for valence electrons, 4 for the number of lone pair of electrons and 4 for the number of shared electrons in equation (1) to calculate the formal charge on first oxygen atom connected to nitrogen.
Substitute 6 for valence electrons, 2 for the number of lone pair of electrons and 6 for the number of shared electrons in equation (1) to calculate the formal charge on second oxygen atom connected to nitrogen.
For structure II:
Substitute 5 for valence electrons, 0 for the number of lone pair of electrons and 8 for the number of shared electrons in equation (1) to calculate the formal charge on nitrogen atom.
Substitute 6 for valence electrons, 2 for the number of lone pair of electrons and 6 for the number of shared electrons in equation (1) to calculate the formal charge on first oxygen atom connected to nitrogen.
Substitute 6 for valence electrons, 4 for the number of lone pair of electrons and 4 for the number of shared electrons in equation (1) to calculate the formal charge on second oxygen atom connected to nitrogen.
The possible resonance structures are as follows:
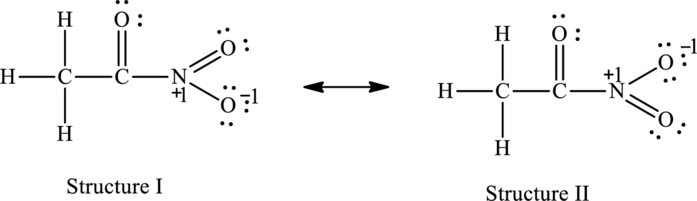
All the structures have same formal charge. Also, the atoms that have charge are same in each structure. Therefore, all structures are equally important.
(b)
Interpretation:
The possible resonance structures for the following skeleton structure have to be determined. Also, the most important resonance structure has to be identified.
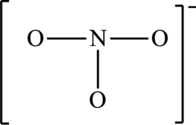
Concept Introduction:
Refer to part (a).
(b)
Answer to Problem 9.68QE
The possible resonance structures are as follows:
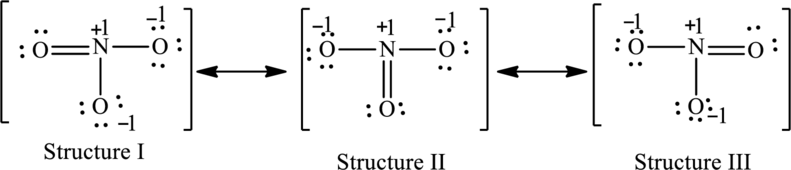
All the structures are equally important.
Explanation of Solution
The skeleton structure is,
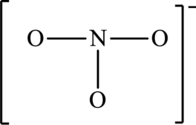
The resonance structures are as follows:
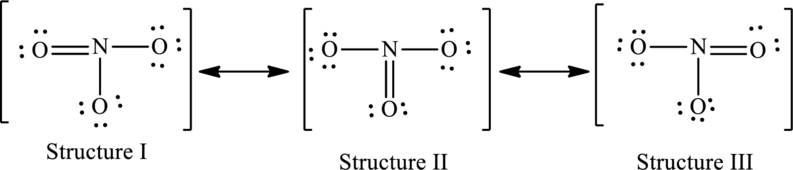
For structure I:
Substitute 5 for valence electrons, 0 for lone pair of electrons and 8 for the number of shared electrons in equation (1) to calculate the formal charge on nitrogen atom.
Substitute 6 for valence electrons, 4 for lone pair of electrons and 4 for the number of shared electrons in equation (1) to calculate the formal charge on first oxygen atom.
Substitute 6 for valence electrons, 6 for lone pair of electrons and 2 for the number of shared electrons in equation (1) to calculate the formal charge on second oxygen atom.
Substitute 6 for valence electrons, 6 for lone pair of electrons and 2 for the number of shared electrons in equation (1) to calculate the formal charge on third oxygen atom.
For structure II:
Substitute 5 for valence electrons, 0 for lone pair of electrons and 8 for the number of shared electrons in equation (1) to calculate the formal charge on nitrogen atom.
Substitute 6 for valence electrons, 6 for lone pair of electrons and 2 for the number of shared electrons in equation (1) to calculate the formal charge on first oxygen atom.
Substitute 6 for valence electrons, 4 for lone pair of electrons and 4 for the number of shared electrons in equation (1) to calculate the formal charge on second oxygen atom.
Substitute 6 for valence electrons, 6 for lone pair of electrons and 2 for the number of shared electrons in equation (1) to calculate the formal charge on third oxygen atom.
For structure III:
Substitute 5 for valence electrons, 0 for lone pair of electrons and 8 for the number of shared electrons in equation (1) to calculate the formal charge on nitrogen atom.
Substitute 6 for valence electrons, 6 for lone pair of electrons and 2 for the number of shared electrons in equation (1) to calculate the formal charge on first oxygen atom.
Substitute 6 for valence electrons, 6 for lone pair of electrons and 2 for the number of shared electrons in equation (1) to calculate the formal charge on second oxygen atom.
Substitute 6 for valence electrons, 4 for lone pair of electrons and 4 for the number of shared electrons in equation (1) to calculate the formal charge on third oxygen atom.
Possible resonance structures are as follows:
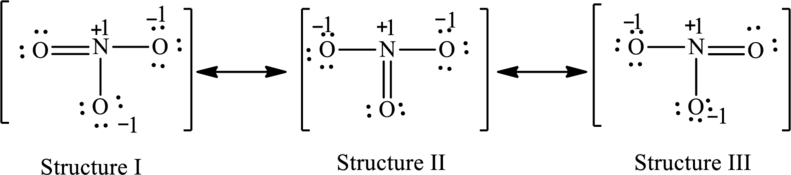
All the structures have the same formal charge. Also, the atoms that have charge are same in each structure. Therefore, all structures are equally important.
Want to see more full solutions like this?
Chapter 9 Solutions
Chemistry Principles And Practice
- Organic Functional Groups entifying positions labeled with Greek letters in acids and derivatives 1/5 ssible, replace an H atom on the a carbon of the molecule in the drawing area with a ce an H atom on the ẞ carbon with a hydroxyl group substituent. ne of the substituents can't be added for any reason, just don't add it. If neither substi er the drawing area. O H OH Oneither substituent can be added. Check D 1 Accessibility ado na witharrow_forwardDifferentiate between electrophilic and nucleophilic groups. Give examples.arrow_forwardAn aldehyde/ketone plus an alcohol gives a hemiacetal, and an excess of alcohol gives an acetal. The reaction is an equilibrium; in aldehydes, it's shifted to the right and in ketones, to the left. Explain.arrow_forward
- Draw a Haworth projection or a common cyclic form of this monosaccharide: H- -OH H- OH H- -OH CH₂OHarrow_forwardAnswer the question in the first photoarrow_forwardGgggffg2258555426855 please don't use AI Calculate the positions at which the probability of a particle in a one-dimensional box is maximum if the particle is in the fifth energy level and in the eighth energy level.arrow_forward
- Draw product A, indicating what type of reaction occurs. NH2 F3C CF3 NH OMe NH2-NH2, ACOH Aarrow_forwardPhotochemical smog is formed in part by the action of light on nitrogen dioxide. The wavelength of radiation absorbed by NO2 in this reaction is 197 nm.(a) Draw the Lewis structure of NO2 and sketch its π molecular orbitals.(b) When 1.56 mJ of energy is absorbed by 3.0 L of air at 20 °C and 0.91 atm, all the NO2 molecules in this sample dissociate by the reaction shown. Assume that each absorbed photon leads to the dissociation (into NO and O) of one NO2 molecule. What is the proportion, in parts per million, of NO2 molecules in this sample? Assume that the sample behaves ideally.arrow_forwardCorrect each molecule in the drawing area below so that it has the skeletal ("line") structure it would have if it were dissolved in a 0.1 M aqueous solution of HCI. If there are no changes to be made, check the No changes box under the drawing area. No changes. HO Explanation Check NH, 2 W O :□ G ©2025 M unter Accessibilityarrow_forward
 Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning
Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax
Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax





