
Concept explainers
Oxymercuration
Concerns about mercury’s toxicity have led to decreased use of mercury-based reagents in
Among the synthetically useful reactions of
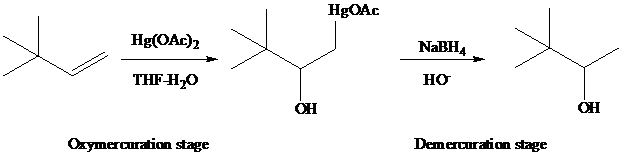
The reaction is performed in two operations, the first of which is oxymercuration. In this stage the alkene is treated with
From the overall reaction, we see that oxymercuration–demercuration
1. accomplishes hydration of the double bond in accordance with Markovnikov’s rule, and
2. carbocation rearrangements do not occur.
Additional information from stereochemical studies with other
3. anti addition of
4. the replacement of
The structure of the intermediate in oxymercuration has received much attention and can beapproached by considering what is likely to happen when the electrophile
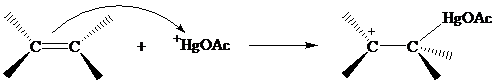
Recall from Section
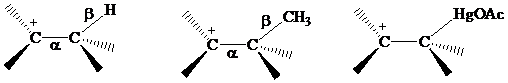
The electrons in a
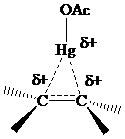
The problems that follow explore various synthetic aspects of oxymercuration–demercuration.Experimental procedures sometimes vary depending on the particular transformation. The source of the electrophile may be a mercury(II) salt other than
Oxymercuration–demercuration of
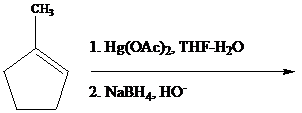
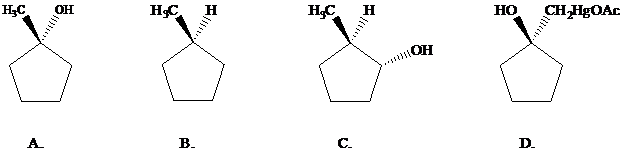
Want to see the full answer?
Check out a sample textbook solution
Chapter 8 Solutions
ORGANIC CHEMISTRY-PACKAGE >CUSTOM<
- +3413 pts /4800 Question 38 of 48 > Write the full electron configuration for a Kion. © Macmillan Learning electron configuration: ↓ Resources Solution Penalized → Al Tutor Write the full electron configuration for an Fion. electron configuration: T G 6 & 7 Y H כ Y 00 8 hp 9 J K no L 144 P 112 | t KC 47°F Clear ins prt sc delete ] backspace erarrow_forwardHow to solve these types of problems step by step? I'm so confused.arrow_forwardIdentify the expected product of the following Claisen rearrangement. || = IV OV 00000 5 ОН Он Он Он Он || III IV Varrow_forward
- Can you please color-code and explain how to solve this and any molecular orbital diagram given? I'm so confused; could you provide baby steps regardless of which problem type they gave me?arrow_forwardConsider the following structure. OH Esmolol The synthesis of this compound uses a building block derived from either ethylene oxide or epichlorohydrin. 1) Determine which building block was used: | 2) Draw the structure of the nucleophiles that were used along with this building block in the synthesis of the molecule. • Draw one structure per sketcher. Add additional sketchers using the drop-down menu in the bottom right corner. You do not have to consider stereochemistry. Θε {n [arrow_forward< 10:44 5GW 10 Question 7/8 Show Answer Convert 46.0 mm to inches (1 inch = 2.54 cm) 46.0 DAM STARTING AMOUNT 1 cm 1 in 46.0 mm x ☑ 10 mm 10 cm ADD FACTOR DELETE x() X × = 1.81 in = 1 10 Dam ANSWER RESET ១ 2.54 0.0460 mm 10 1000 in 0.001 11.7 m 4.60 18.1 cm 100 1.81 0.394 1 0.1 46.0 0.01 Tap here for additional resourcesarrow_forward
- < 10:44 Question 6/8 5GW (10 Submit A cake recipe calls for 230.0 mL of buttermilk. How 230.0 many cups is this? DAL STARTING AMOUNT × 1 cups 230.0 mL x = 0.9722 cups 230.0 mL ADD FACTOR DELETE (( ) = 1 cups 230.0 DAE ANSWER RESET ១ 9.722 × 105 0.8706 cups 8.706 × 104 1 L 8.706 × 105 0.9722 quart 10 100 mL 0.001 0.1 6.076 × 103 0.01 9.722 × 104 230.0 0.06076 4 1.0567 1000 6.076 × 104 Tap here for additional resourcesarrow_forward< 10:44 Question 6/8 5GW (10 Submit A cake recipe calls for 230.0 mL of buttermilk. How 230.0 many cups is this? DAL STARTING AMOUNT × 1 cups 230.0 mL x = 0.9722 cups 230.0 mL ADD FACTOR DELETE (( ) = 1 cups 230.0 DAE ANSWER RESET ១ 9.722 × 105 0.8706 cups 8.706 × 104 1 L 8.706 × 105 0.9722 quart 10 100 mL 0.001 0.1 6.076 × 103 0.01 9.722 × 104 230.0 0.06076 4 1.0567 1000 6.076 × 104 Tap here for additional resourcesarrow_forwardShow work in detailed of all the options. Don't give Ai generated solutionarrow_forward
- Predict the Product. Predict the major organic product for the following reaction:arrow_forwardPlease provide the complete mechanism for the reaction below including arrows, intermediates, and formal charges.arrow_forwardCan you please explain this to me? Maybe color-code it in essence and highlight it.arrow_forward

 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning


